Abstract
Objective
Pain assessment usually involves the use of subjective pain scales; as their use may be associated with inter-/intra-observer bias, objective pain measurements, such as assessment of cortisol response to pain, are needed. This study aimed to compare the efficacy of oral dextrose and a pacifier in neonatal pain control using an objective measurement of salivary cortisol level and subjective pain scoring.
Methods
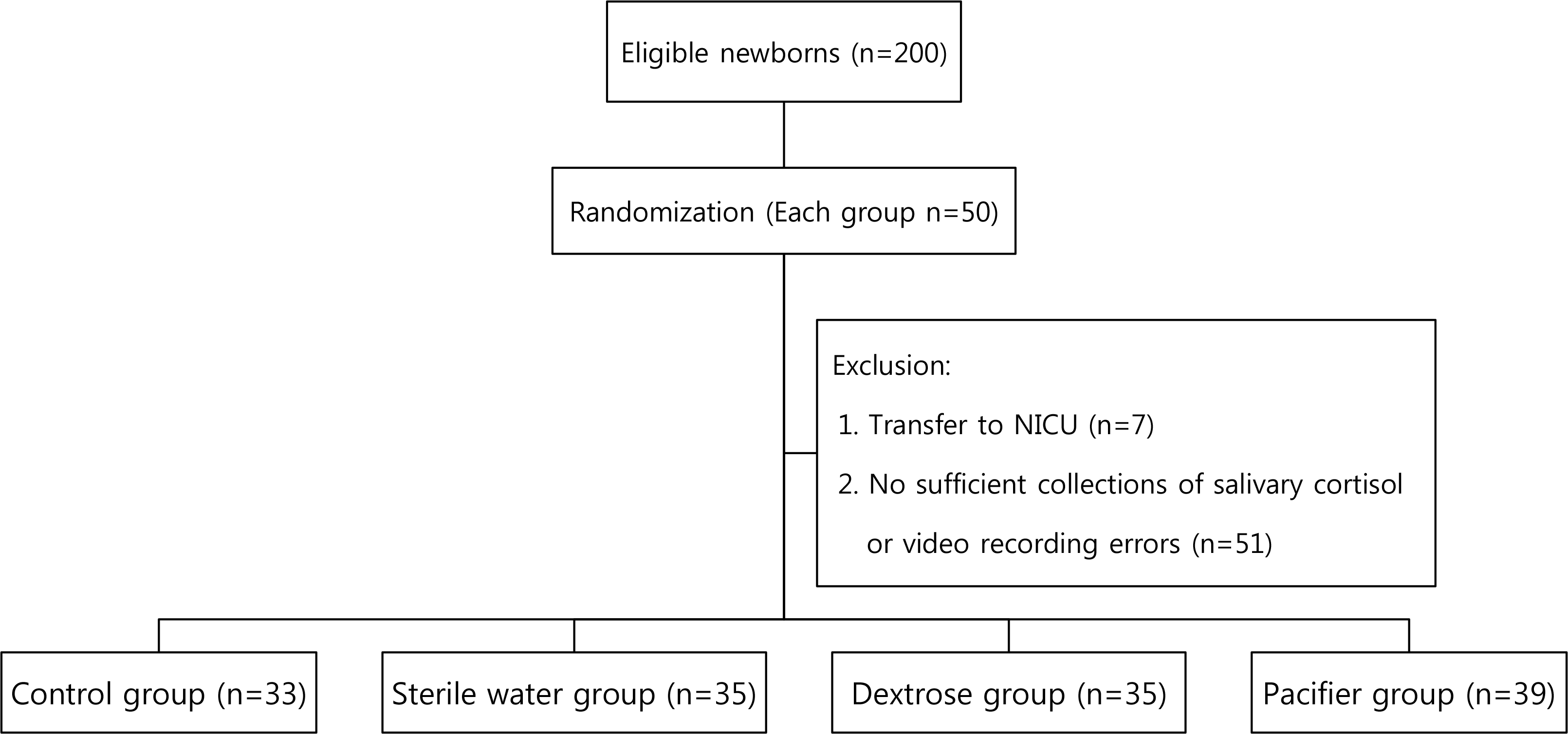
This prospective, randomized, partially blinded clinical trial included healthy newborns from a nursery (n=142). Blood was sampled using a lancet and newborns were randomly assigned to four groups by drawing lots: control (n=33), sterile water (n=35), 25% dextrose (n=35), and pacifier group (n=39). For all groups, neonatal infant pain scale, neonatal facial coding system, and premature infant pain profile scores were evaluated before, during, and 2 minutes after newborn screening test by two independent observers who watched recorded videos. Moreover, samples of saliva were collected before and 30 minutes after the pain procedure, and salivary cortisol level was measured using an enzyme-linked immunosorbent assay.
REFERENCES
1). Anand KJ., Aranda JV., Berde CB., Buckman S., Capparelli EV., Carlo W, et al. Summary proceedings from the neonatal pain-control group. Pediatrics. 2006. 117(3 Pt 2):S9–22.

2). Peterson BS., Vohr B., Staib LH., Cannistraci CJ., Dolberg A., Schneider KC, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000. 284:1939–47.

3). Hall RW., Anand KJS. Short- and long-term impact of neonatal pain and stress: more than an ouchie. NeoReviews. 2005. 6:e69–75.
4). Witt N., Coynor S., Edwards C., Bradshaw H. A guide to pain assessment and management in the neonate. Curr Emerg Hosp Med Rep. 2016. 4:1–10.

5). Slater R., Cantarella A., Franck L., Meek J., Fitzgerald M. How well do clinical pain assessment tools reflect pain in infants? PLoS Med. 2008. 5:e129.

6). Smith GC., Gutovich J., Smyser C., Pineda R., Newnham C., Tjoeng TH, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 2011. 70:541–9.

7). COMMITTEE ON FETUS and NEWBORN and SECTION ON ANESTHE-SIOLOGY AND PAIN MEDICINE. Prevention and management of procedural pain in the neonate: an update. Pediatrics. 2016. 137:e20154271.
8). Lago P., Garetti E., Bellieni CV., Merazzi D., Savant Levet P., Ancora G, et al. Systematic review of nonpharmacological analgesic interventions for common needle-related procedure in newborn infants and development of evidence-based clinical guidelines. Acta Paediatr. 2017. 106:864–70.
9). Spence K., Henderson-Smart D., New K., Evans C., Whitelaw J., Woolnough R, et al. Evidenced-based clinical practice guideline for management of newborn pain. J Paediatr Child Health. 2010. 46:184–92.

10). Lawrence J., Alcock D., McGrath P., Kay J., MacMurray SB., Dulberg C. The development of a tool to assess neonatal pain. Neonatal Netw. 1993. 12:59–66.

11). Grunau RV., Craig KD. Pain expression in neonates: facial action and cry. Pain. 1987. 28:395–410.

12). Stevens B., Johnston C., Petryshen P., Taddio A. Premature infant pain profile: development and initial validation. Clin J Pain. 1996. 12:13–22.

13). Shah VS., Ohlsson A. Venepuncture versus heel lance for blood sampling in term neonates. Cochrane Database Syst Rev. 2011. 10:CD001452.

14). Khurana S., Hall RW., Anand KJS. Treatment of pain and stress in the neonate: when and how. NeoReviews. 2005. 6:e76–86.
15). Mörelius E., Theodorsson E., Nelson N. Salivary cortisol and mood and pain profiles during skin-to-skin care for an unselected group of mothers and infants in neonatal intensive care. Pediatrics. 2005. 116:1105–13.
16). Mörelius E., Theodorsson E., Nelson N. Stress at three-month immunization: parents' and infants' salivary cortisol response in relation to the use of pacifier and oral glucose. Eur J Pain. 2009. 13:202–8.

17). South MM., Strauss RA., South AP., Boggess JF., Thorp JM. The use of nonnutritive sucking to decrease the physiologic pain response during neonatal circumcision: a randomized controlled trial. Am J Obstet Gynecol. 2005. 193:537–42. discussion 542-3.

18). Mörelius E., He HG., Shorey S. Salivary cortisol reactivity in preterm infants in neonatal intensive care: an integrative review. Int J Environ Res Public Health. 2016. 13:337.

19). Mörelius E., Broström EB., Westrup B., Sarman I., Örtenstrand A. The Stockholm neonatal family-centered care study: effects on salivary cortisol in infants and their mothers. Early Hum Dev. 2012. 88:575–81.

20). Herrington CJ., Olomu IN., Geller SM. Salivary cortisol as indicators of pain in preterm infants: a pilot study. Clin Nurs Res. 2004. 13:53–68.
21). Gunnar MR., Hertsgaard L., Larson M., Rigatuso J. Cortisol and behavioral responses to repeated stressors in the human newborn. Dev Psychobiol. 1991. 24:487–505.

23). Gunnar MR., Malone S., Vance G., Fisch RO. Coping with aversive stimulation in the neonatal period: quiet sleep and plasma cortisol levels during recovery from circumcision. Child Dev. 1985. 56:824–34.

24). Ramsay DS., Lewis M. The effects of birth condition on infants' cortisol response to stress. Pediatrics. 1995. 95:546–9.

25). Naughton KA. The combined use of sucrose and nonnutritive sucking for procedural pain in both term and preterm neonates: an integrative review of the literature. Adv Neonatal Care. 2013. 13:9–19. quiz 20-1.
26). Stevens B., Johnston C., Franck L., Petryshen P., Jack A., Foster G. The efficacy of developmentally sensitive interventions and sucrose for relieving procedural pain in very low birth weight neonates. Nurs Res. 1999. 48:35–43.

27). Akman I., Ozek E., Bilgen H., Ozdogan T., Cebeci D. Sweet solutions and pacifiers for pain relief in newborn infants. J Pain. 2002. 3:199–202.

28). Gibbins S., Stevens B., Hodnett E., Pinelli J., Ohlsson A., Darlington G. Efficacy and safety of sucrose for procedural pain relief in preterm and term neonates. Nurs Res. 2002. 51:375–82.

Table 1.
Clinical Characteristics of Newborns
Table 2.
Neonatal Pain Scores according to Analgesic Procedures
Table 3.
Comparison of Score Changes of Neonatal Infant Pain Scale and Neonatal Facial Coding System from Baseline to Injection and Recovery
| NIPS scores∗ | NFCS scores† | |||
|---|---|---|---|---|
| Δ Pain‡ | Δ Recovery§ | Δ Pain‡ | Δ Recovery§ | |
| Control group | 3.7±2.0 | 2.4±2.2 | 4.5±2.2 | 2.9±2.6 |
| Sterile water group | 3.0±2.1 | 1.9±2.8 | 4.1±2.4 | 2.3±2.9 |
| Dextrose group | 3.8±1.9 | 1.8±3.0 | 4.8±2.1 | 2.4±3.0 |
| Pacifier group | 3.7±2.3 | 3.0±2.5 | 4.9±2.6 | 3.6±2.8 |
Values are presented as mean±standard deviation. Abbreviations: NIPS, Neonatal Infant Pain Scores; NFCS, Neonatal Facial Coding System Scores.
Table 4.
Comparison of Cortisol Levels and Changes of Cortisol Levels from Baseline to Pain
| Baseline (µg/dL) | Pain (µg/dL) Δ Pain∗ | P value† | |
|---|---|---|---|
| Control group | 1.260±1.121 | 2.156±1.908 0.897±1.567 | Reference |
| Sterile water group | 1.240±0.958 | 1.553±1.060 0.313±1.181 | 0.229 |
| Dextrose group | 1.194±1.281 | 1.246±0.959 0.052±1.577 | 0.046 |
| Pacifier group | 1.162±1.434 | 1.532±1.380 0.369±1.430 | 0.284 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download