This article has been
cited by other articles in ScienceCentral.
Abstract
The aim of this study was to examine the daily practice patterns of Symbicort® Maintenance and Reliever Therapy (SMART) in Korean asthmatic patients and to analyze clinical signs related to overuse. This study used an observational, multicenter, noninterventional, prospective, uncontrolled design for examining asthmatic patients prescribed SMART to assess the frequency and pattern of Symbicort® usage as a maintenance and reliever medication. The characteristics of patients showing signs of overuse (frequency of inhalation: 8 or more times per day) were also analyzed. Among the 1,518 patients analyzed, 1,292 (85.1%) completed the trial. The number of mean inhalations per day was 2.14±1.15; the number of patients who had at least 1 as needed usage (PRN) inhalation per day was 843 (55.5%); the mean frequency of PRN use was 0.25±0.67 inhalations per day. The number of patients who overused for at least 1 day was 260 (17.1%). In particular, young patients, patients with limited physical activity, and patients with nocturnal symptoms demonstrated high frequency of overuse. The frequency of overuse during SMART was not high in Korean asthmatic patients and the asthma status of follow-up outpatients improved overall. However, there is a need for careful education targeted toward younger patients, patients with limited physical activity, and patients with nocturnal symptoms owing to their tendency to frequently overuse.
Go to :

Keywords: Symbicort® maintenance and reliever therapy, budesonide, formoterol, prescription drug overuse, asthma
INTRODUCTION
The combination of inhaled corticosteroid (ICS) and long-acting beta-2 agonist (LABA) is currently widely used for asthma patients whose symptoms cannot be controlled with ICS alone. Among the various ICS/LABA combinations, the budesonide/formoterol inhaler can be used for maintenance therapy as well as for both maintenance and reliever therapy. For example, budesonide/formoterol maintenance and reliever therapy has been shown to efficiently prevent exacerbation of asthma.
12345 Asthma exacerbation usually denotes worsening of asthma symptoms that requires intense management to prevent further deterioration.
6 In the study by Kuna et al.,
3 budesonide/formoterol maintenance and reliever therapy showed a 39% decrease in severe asthma exacerbations compared to the regimen consisting of inhaling a fixed dose of salmeterol/fluticasone. This preventive activity is based on the effects of both budesonide and formoterol; budesonide suppresses inflammation in early stages of exacerbation and formoterol provides rapid symptomatic relief by expanding the airway smooth muscle.
7 Budesonide/formoterol maintenance and reliever therapy is recommended for use in asthmatic patients in the current Global Initiative for Asthma (GINA) guideline
8 and in local guidelines in various countries. Despite achieving the treatment goal for asthma, there has been limited data on the under- and overuse of budesonide/formoterol maintenance and reliever therapy in real-life settings. In comparison to traditional treatment strategy, which typically uses a fixed-dose ICS/LABA combination as a maintenance treatment and a short-acting beta-2 adrenoceptor agonist (SABA) as a reliever, budesonide/formoterol maintenance and reliever therapy has been the subject of concern due to the possible under- and overuse of the budesonide/formoterol combination. Despite such concerns, safety issues associated with budesonide/formoterol maintenance and reliever therapy have not been reported in previous clinical trials.
910 Moreover, prospective clinical trials conducted to address safety concerns did not show any cases of overuse during budesonide/formoterol maintenance and reliever therapy.
11 However, these results were obtained from patients who met inclusion criteria, so that they do not sufficiently reflect real-life scenarios. Therefore, more data are needed to understand how asthmatic patients actually use budesonide/formoterol maintenance and reliever therapy in real life.
12 Accordingly, the present study aimed to investigate the use of budesonide/formoterol maintenance and reliever therapy in real-life settings.
Go to :

MATERIALS AND METHODS
This 6-month study used an observational, multicenter, prospective, uncontrolled design with follow-up. The study was conducted in 82 centers throughout Korea between July 2007 and June 2008. The study participants included patients diagnosed with asthma on the basis of clinical manifestations or diagnostic test results and were being treated with Symbicort® (AstraZeneca, Seoul, Korea; a combination of 160 µg budesonide and 4.5 µg formoterol) Maintenance and Reliever Therapy (SMART). Patients who had participated in any other clinical trials at the time of enrollment were excluded. The study received approval from the Institutional Review Board of each relevant institution. To be eligible for the study, patients were required to give informed consent. Demographic information, gender, age, maintenance dose, start date of Symbicort®, asthma control status, and concomitant asthma medication use were recorded on an electronic case report form (e-CRF). All patients received education on usage of SMART; relevant instruction manuals were also distributed. Up to 6 months, i.e., until the end of the study, information on the total daily use of Symbicort® (maintenance and reliever use) for the previous day was obtained every day from each patient by using an automated calling service (ACS) and short message service (SMS).
At the end of the trial, we obtained information on whether SMART would be continued, whether the reason(s) for discontinuation (in case) was gathered, and whether the information on asthma control status and concomitant asthma medications for follow-up outpatients was re-recorded. The diagnosis of asthma was confirmed at the time of enrollment and patients were treated with conventional methods. We also recorded cases where changes were made to the Symbicort® prescription.
The frequency of “as needed usage (PRN)” of Symbicort® as a reliever therapy was calculated by subtracting the prescribed maintenance from the total daily usage. For adult and adolescent patients aged 12 years and more, the recommended dosage of Symbicort® 160/4.5 µg is 2 inhalations once daily or 1 inhalation twice daily, and overuse is defined as daily usage frequency of 8 or more inhalations.
1314 Patients with more than 8 inhalations daily should be strongly recommended to seek medical advice, although a total daily dose of up to 12 inhalations could be used for a limited period.
13
Go to :

RESULTS
Of the 1,799 total participants, analysis was performed on data from 1,518 using Symbicort® 160/4.5 µg, whereas 71 who were given Symbicort® 80/4.5 µg, 68 who did not receive SMART education and/or the instruction manual, and 142 who did not use the ACS and SMS, were excluded (
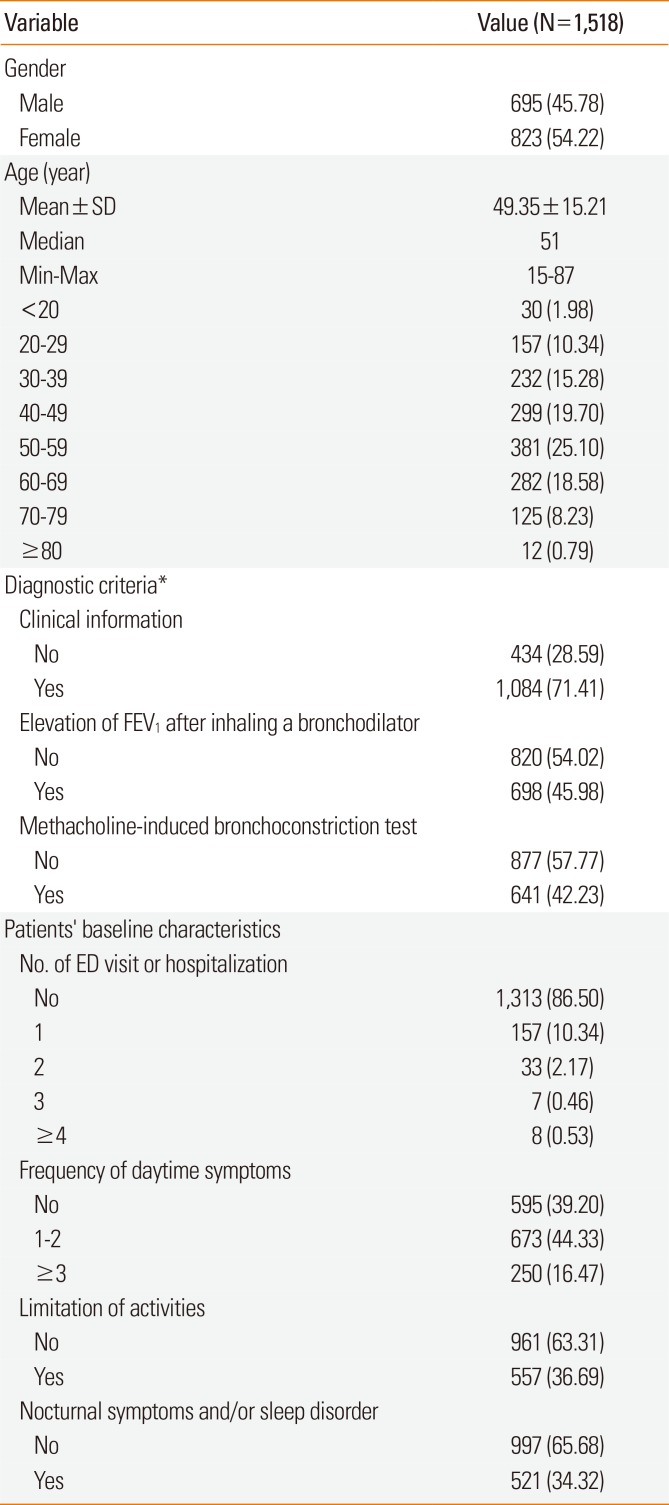
Fig. 1). Of 1,518 participants included in this study, 1,292 (85.1%) completed the study. Baseline characteristics and concomitant medication are summarized in
Tables 1 and
2.
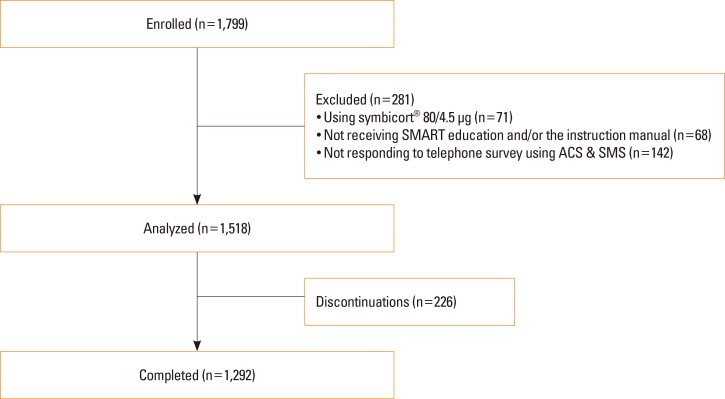 | Fig. 1Study design. ACS, automated calling service; SMART, Symbicort® Maintenance and Reliever Therapy; SMS, short message service.
|
Table 1
Selected demographics and participant clinical characteristics
|
Variable |
Value (N=1,518) |
|
Gender |
|
|
Male |
695 (45.78) |
|
Female |
823 (54.22) |
|
Age (year) |
|
|
Mean±SD |
49.35±15.21 |
|
Median |
51 |
|
Min-Max |
15–87 |
|
<20 |
30 (1.98) |
|
20–29 |
157 (10.34) |
|
30–39 |
232 (15.28) |
|
40–49 |
299 (19.70) |
|
50–59 |
381 (25.10) |
|
60–69 |
282 (18.58) |
|
70–79 |
125 (8.23) |
|
≥80 |
12 (0.79) |
|
Diagnostic criteria*
|
|
|
Clinical information |
|
|
No |
434 (28.59) |
|
Yes |
1,084 (71.41) |
|
Elevation of FEV1 after inhaling a bronchodilator |
|
|
No |
820 (54.02) |
|
Yes |
698 (45.98) |
|
Methacholine-induced bronchoconstriction test |
|
|
No |
877 (57.77) |
|
Yes |
641 (42.23) |
|
Patients' baseline characteristics |
|
|
No. of ED visit or hospitalization |
|
|
No |
1,313 (86.50) |
|
1 |
157 (10.34) |
|
2 |
33 (2.17) |
|
3 |
7 (0.46) |
|
≥4 |
8 (0.53) |
|
Frequency of daytime symptoms |
|
|
No |
595 (39.20) |
|
1–2 |
673 (44.33) |
|
≥3 |
250 (16.47) |
|
Limitation of activities |
|
|
No |
961 (63.31) |
|
Yes |
557 (36.69) |
|
Nocturnal symptoms and/or sleep disorder |
|
|
No |
997 (65.68) |
|
Yes |
521 (34.32) |

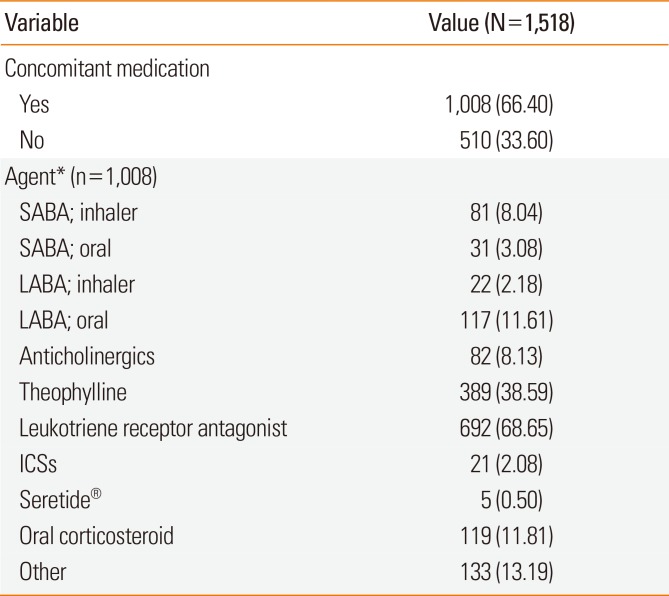
Table 2
Summary of concomitant medication usage
|
Variable |
Value (N=1,518) |
|
Concomitant medication |
|
|
Yes |
1,008 (66.40) |
|
No |
510 (33.60) |
|
Agent* (n=1,008) |
|
|
SABA; inhaler |
81 (8.04) |
|
SABA; oral |
31 (3.08) |
|
LABA; inhaler |
22 (2.18) |
|
LABA; oral |
117 (11.61) |
|
Anticholinergics |
82 (8.13) |
|
Theophylline |
389 (38.59) |
|
Leukotriene receptor antagonist |
692 (68.65) |
|
ICSs |
21 (2.08) |
|
Seretide® |
5 (0.50) |
|
Oral corticosteroid |
119 (11.81) |
|
Other |
133 (13.19) |

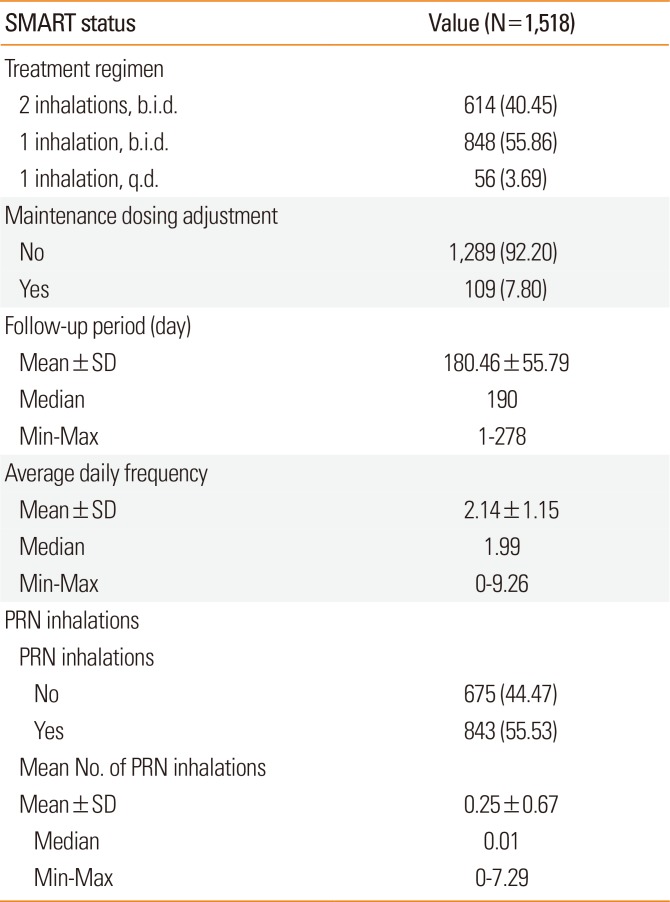
The most frequently prescribed maintenance dose of Symbicort® was single inhalation, 2 times a day (b.i.d.; n=848, 55.9%) (
Table 3). There were 109 cases (7.8%) in which the maintenance dose was changed during the study period. The mean follow-up period during the study was 180.46±55.79 days. Analysis by age group showed that the participants in the 30-39 years age group had the highest mean number of response days, while those in the <20 and ≥60 age groups showed a relatively lower mean number of response days (
P=0.012).
Table 3
Summary of SMART usage
|
SMART status |
Value (N=1,518) |
|
Treatment regimen |
|
|
2 inhalations, b.i.d. |
614 (40.45) |
|
1 inhalation, b.i.d. |
848 (55.86) |
|
1 inhalation, q.d. |
56 (3.69) |
|
Maintenance dosing adjustment |
|
|
No |
1,289 (92.20) |
|
Yes |
109 (7.80) |
|
Follow-up period (day) |
|
|
Mean±SD |
180.46±55.79 |
|
Median |
190 |
|
Min–Max |
1–278 |
|
Average daily frequency |
|
|
Mean±SD |
2.14±1.15 |
|
Median |
1.99 |
|
Min–Max |
0–9.26 |
|
PRN inhalations |
|
|
PRN inhalations |
|
|
No |
675 (44.47) |
|
Yes |
843 (55.53) |
|
Mean No. of PRN inhalations |
|
|
Mean±SD |
0.25±0.67 |
|
Median |
0.01 |
|
Min–Max |
0–7.29 |

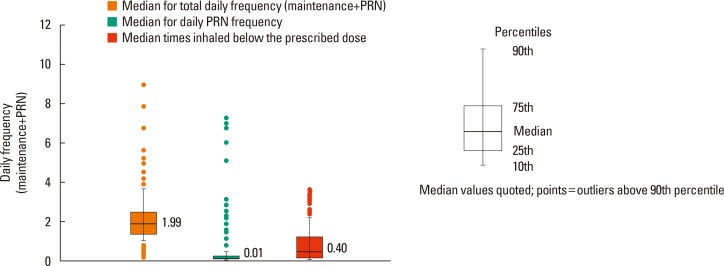
The mean number of daily Symbicort® inhalations during the study period was 2.14±1.15 (
Fig. 2). Participants who inhaled PRN were 843 (55.5%). The mean number of PRN inhalations for all participants was 0.25±0.67 (median=0.01) (
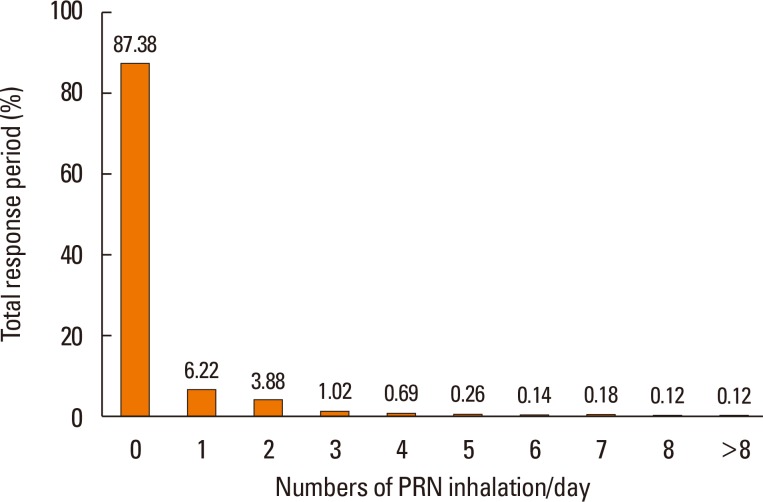
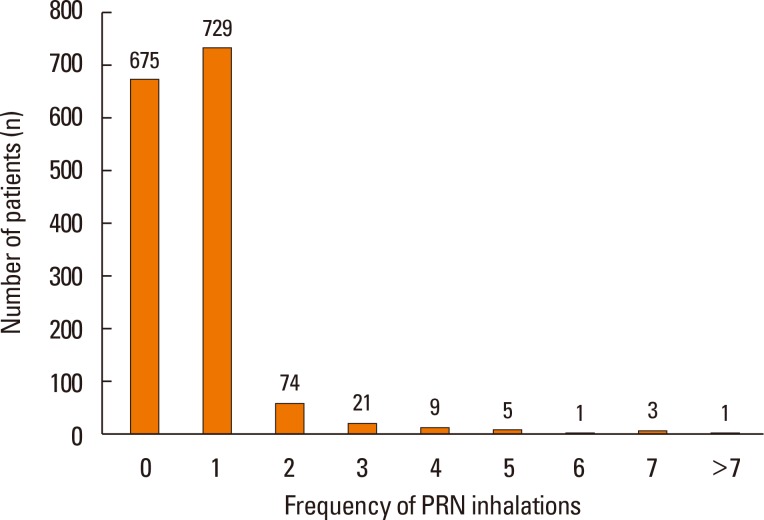
Table 3). The results demonstrated that most participants (92.5%) required a mean of one or fewer PRN inhalations per day. In total response period, no PRN inhalation use was 87.4% and more than 5 times reliever use per day was less than 1% in total period (
Fig. 4). The mean number of PRN inhalations per day was significantly higher in patients taking concomitant medication than in those not taking concomitant medication (0.28±0.73 vs 0.20±0.53,
P=0.018).
 | Fig. 2Frequency of inhalations using SMART. SMART, Symbicort® Maintenance and Reliever Therapy; PRN, as needed usage.
|
 | Fig. 4Distribution of response period by numbers of PRN inhalation, PRN as needed usage.
|
Of the days on which the participants responded, the number of participants who inhaled Symbicort® less than the prescribed number was 1,341 (88.3%). The mean number of times Symbicort® was inhaled less than the prescribed number was 0.88±1.02 (median=0.40). The participants who inhaled >0 and ≤1 time less than prescribed dose were most common (n=825, 54.4%). Only 11% (n=177) of the participants complied with the prescribed usage. Of the days on which the participants responded, the mean percentage of days on which the participants inhaled in compliance with the prescribed usage (having no days of under-inhalation) was 56.95%±41.04%.
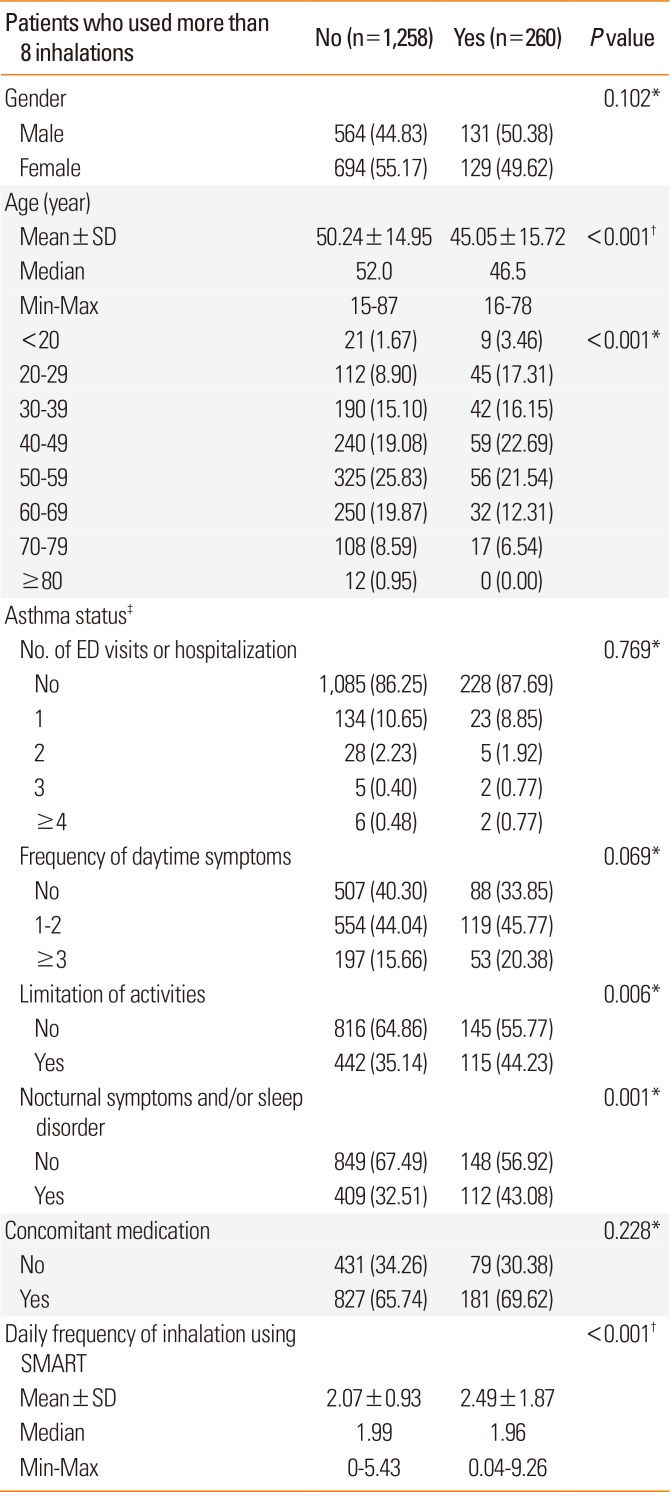
Investigation of overuse (defined as exceeding 8 inhalations per day) showed that 260 participants (17.1%) overused the medication at least for 1 day. The mean number of days of overuse was 5.94±18.91 (median=1 day). Among the overusing patients, 58.5% inhaled more than 8 times for 1 day only and approximately 90% overused for less than 1 week.
Table 4 shows the main characteristics of participants who overused Symbicort®. There were no differences between the 2 groups with respect to diagnostic methods for asthma, number of emergency department (ED) visits and hospitalizations, and daytime symptoms. However, the overuse group had a lower mean age with higher percentage of patients in the 20-29 age group (
P<0.001). The overuse group also showed higher rates of limited physical activities (
P<0.05) and nocturnal symptoms and/or sleep disorder (
P<0.001).
Table 4
Demographics and clinical characteristics of patients with overuse
|
Patients who used more than 8 inhalations |
No (n=1,258) |
Yes (n=260) |
P value |
|
Gender |
|
|
0.102*
|
|
Male |
564 (44.83) |
131 (50.38) |
|
Female |
694 (55.17) |
129 (49.62) |
|
Age (year) |
|
|
|
|
Mean±SD |
50.24±14.95 |
45.05±15.72 |
<0.001†
|
|
Median |
52.0 |
46.5 |
|
|
Min–Max |
15–87 |
16–78 |
|
|
<20 |
21 (1.67) |
9 (3.46) |
<0.001*
|
|
20–29 |
112 (8.90) |
45 (17.31) |
|
|
30–39 |
190 (15.10) |
42 (16.15) |
|
|
40–49 |
240 (19.08) |
59 (22.69) |
|
|
50–59 |
325 (25.83) |
56 (21.54) |
|
|
60–69 |
250 (19.87) |
32 (12.31) |
|
|
70–79 |
108 (8.59) |
17 (6.54) |
|
|
≥80 |
12 (0.95) |
0 (0.00) |
|
|
Asthma status‡
|
|
|
|
|
No. of ED visits or hospitalization |
|
|
0.769*
|
|
No |
1,085 (86.25) |
228 (87.69) |
|
1 |
134 (10.65) |
23 (8.85) |
|
2 |
28 (2.23) |
5 (1.92) |
|
3 |
5 (0.40) |
2 (0.77) |
|
≥4 |
6 (0.48) |
2 (0.77) |
|
Frequency of daytime symptoms |
|
|
0.069*
|
|
No |
507 (40.30) |
88 (33.85) |
|
1–2 |
554 (44.04) |
119 (45.77) |
|
≥3 |
197 (15.66) |
53 (20.38) |
|
Limitation of activities |
|
|
0.006*
|
|
No |
816 (64.86) |
145 (55.77) |
|
Yes |
442 (35.14) |
115 (44.23) |
|
Nocturnal symptoms and/or sleep disorder |
|
|
0.001*
|
|
No |
849 (67.49) |
148 (56.92) |
|
Yes |
409 (32.51) |
112 (43.08) |
|
Concomitant medication |
|
|
0.228*
|
|
No |
431 (34.26) |
79 (30.38) |
|
Yes |
827 (65.74) |
181 (69.62) |
|
Daily frequency of inhalation using SMART |
|
|
<0.001†
|
|
Mean±SD |
2.07±0.93 |
2.49±1.87 |
|
Median |
1.99 |
1.96 |
|
Min–Max |
0–5.43 |
0.04–9.26 |

At the end of the trial, of the 1,163 participants who attended through outpatient visits and telephone responses, 1,096 continued SMART, and 1,036 were followed up on an outpatient basis and monitored for 6 months. An investigation into whether these patients were using other concomitant asthma medications along with Symbicort® showed that 525 patients (52.3%) were using concomitant medication, while 479 patients (47.7%) were not. The most common concomitant medications were leukotriene receptor antagonists (n=362, 69.0%) and theophylline (n=148, 28.2%), while 24 patients (4.6%) were using oral steroids. Despite being instructed to take Symbicort® as a reliever, 53 patients (10.1%) were using additional SABAs.
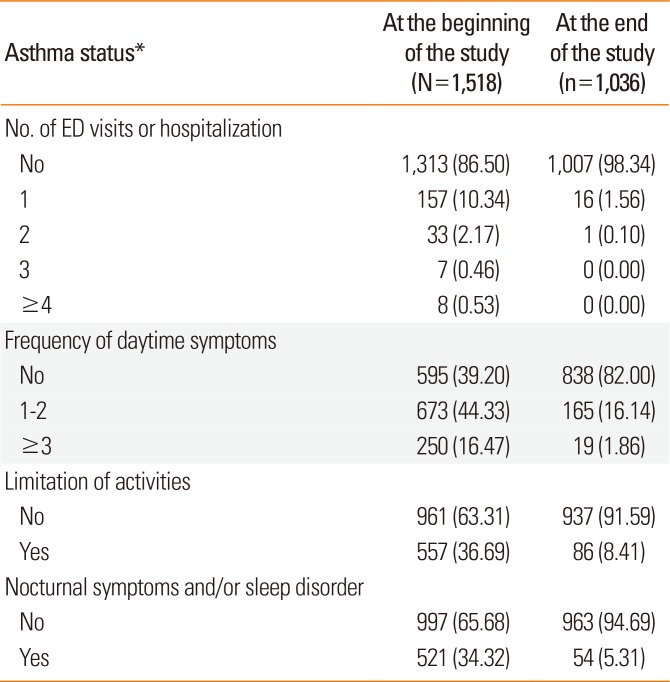
Analysis of asthma status at the end of the study showed improvement in the number of ED visits and hospitalizations, frequency of daytime symptoms, limitation of activities, and nocturnal symptoms and/or sleep disorder, as compared to the data recorded at the beginning of the study (
Table 5).
Table 5
Change of asthma and clinical status of participants at the end of the trial
|
Asthma status*
|
At the beginning of the study (N=1,518) |
At the end of the study (n=1,036) |
|
No. of ED visits or hospitalization |
|
|
|
No |
1,313 (86.50) |
1,007 (98.34) |
|
1 |
157 (10.34) |
16 (1.56) |
|
2 |
33 (2.17) |
1 (0.10) |
|
3 |
7 (0.46) |
0 (0.00) |
|
≥4 |
8 (0.53) |
0 (0.00) |
|
Frequency of daytime symptoms |
|
|
|
No |
595 (39.20) |
838 (82.00) |
|
1–2 |
673 (44.33) |
165 (16.14) |
|
≥3 |
250 (16.47) |
19 (1.86) |
|
Limitation of activities |
|
|
|
No |
961 (63.31) |
937 (91.59) |
|
Yes |
557 (36.69) |
86 (8.41) |
|
Nocturnal symptoms and/or sleep disorder |
|
|
|
No |
997 (65.68) |
963 (94.69) |
|
Yes |
521 (34.32) |
54 (5.31) |

Go to :

DISCUSSION
Despite demonstrated benefits of SMART in many trials,
3151617 safety concerns regarding possible under- and overuse of ICS and beta-2 adrenoceptor agonists have not yet been fully addressed. Accordingly, this study aimed to identify the daily usage of SMART in real-life settings for asthmatic patients in Korea and to analyze overuse-related clinical features.
In this study, the mean daily frequency of PRN inhalations for symptom relief was below 1 inhalation per day (0.25±0.67), and although 17% of the patients took more than 8 inhalations per day, 90% of these patients overused for less than 7 days. The frequency of overuse was significantly higher in patients who were younger and had limited physical activities, nocturnal symptoms, or sleep disorder. According to a recent randomized controlled trial (RCT) conducted in New Zealand, the patient group on SMART used approximately 1.22 times higher cumulative ICS dose than the patient group using a fixed-dose method over the same period; however, SMART showed a significant reduction in the oral corticosteroid dose.
11 Results of the present real-life study showed that the frequency of overuse associated with SMART was not high. The percentage of patients who inhaled Symbicort® in compliance with the prescribed usage was 11%, whereas 34% of the patients used fewer than prescribed inhalations per day. Importantly, only a minority of them missed more than 1 inhalation per day.
At the end of the present study, the asthma status of follow-up outpatients showed a tendency to improve the number of ED visits and hospitalizations, the number of daytime symptoms, limitation of activities, and nocturnal symptoms and/or sleep disorder compared to early stages of treatment. Moreover, most of these patients were continuing with SMART. The percentage of patients who complied with their prescribed dose was lower in this study than in previous studies,
18 which can be due to overall improvement in the patient's asthma status from using SMART. The lower compliance rate observed in this study may also have been due to lack of provision of sufficient education to the patients on inhaler use, because the study was designed as a non-interventional observational one to measure the compliance rate via audience response system (ARS).
As a non-interventional and non-controlled study, this study has some limitations. First, it did not evaluate changes in Symbicort® dosage according to changes in daytime/nocturnal symptoms or changes in symptoms control. Secondly, asthma diagnosis was based on clinical manifestations and diagnostic methods may have differed between medical institutions. Generally, in Korea, asthma diagnosis and drug prescriptions are carried out in primary medical institutions, where utilization of pulmonary function test devices is very low. Therefore, the diagnostic criteria used in the study were determined to be acceptable. Thirdly, this study was unable to evaluate the impact of the patient's level of understanding about SMART overuse. The fact that the response rates were lower in patients younger than 20 or older than 60 years compared to other age groups indicated that there might have been some factors that did not allow these age groups to actively respond to ARS, and thus assessment on this matter is deemed necessary. Further well-designed and large-scale studies are needed to verify whether SMART can be truly helpful in improving drug compliance in Korea.
In this study, daily frequency of overusing inhalers during SMART was not high, which confirmed that SMART in real-life settings would be effective in asthma control without concerns of overuse. However, because the younger age group and patients with limitation of activities and nocturnal symptoms did show a high possibility of overuse, closer attention should be paid to these age groups. Additionally, education for proper inhaler use is expected to be helpful in preventing misuse.
Go to :

ACKNOWLEDGMENTS
All authors equally contributed to concept and design of this study, data acquisition, analysis, and interpretation, and writing of the manuscript. Authors thank all the staff and participants of this study for their important contributions. This study was supported by AstraZeneca Korea, Seoul, Korea.
The present study protocol was reviewed and approved by the institutional review board of Hanyang University Hospital (Reg. No. 2008-R-28). Informed consent was submitted by all subjects when they were enrolled.
Go to :

Notes
Go to :

References
1. O'Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005; 171:129–136. PMID:
15502112.
2. Bousquet J, Boulet LP, Peters MJ, Magnussen H, Quiralte J, Martinez-Aguilar NE, et al. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir Med. 2007; 101:2437–2446. PMID:
17905575.

3. Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martínez-Jimenez NE, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007; 61:725–736. PMID:
17362472.

4. Rabe KF, Pizzichini E, Ställberg B, Romero S, Balanzat AM, Atienza T, et al. Budesonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma: a randomized, double-blind trial. Chest. 2006; 129:246–256. PMID:
16478838.
5. Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet. 2006; 368:744–753. PMID:
16935685.

6. Park HW, Tantisira KG. Genetic signatures of asthma exacerbation. Allergy Asthma Immunol Res. 2017; 9:191–199. PMID:
28293925.

7. Barnes PJ. Scientific rationale for using a single inhaler for asthma control. Eur Respir J. 2007; 29:587–595. PMID:
17329493.

8. Global Initiative for Asthma. Global strategy for asthma management and prevention 2016 [Internet]. [place unknown]: Global Initiative for Asthma;2016. cited 2016 Nov 8. Available from:
http://www.ginasthma.org.
9. Sears MR, Radner F. Safety of budesonide/formoterol maintenance and reliever therapy in asthma trials. Respir Med. 2009; 103:1960–1968. PMID:
19815402.

10. Cates CJ, Lasserson TJ. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events. Cochrane Database Syst Rev. 2010; CD007694. PMID:
20091646.

11. Patel M, Pilcher J, Pritchard A, Perrin K, Travers J, Shaw D, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerbations: a randomised controlled trial. Lancet Respir Med. 2013; 1:32–42. PMID:
24321802.

12. Ställberg B, Naya I, Ekelund J, Eckerwall G. Real-life use of budesonide/formoterol in clinical practice: a 12-month follow-up assessment in a multi-national study of asthma patients established on single-inhaler maintenance and reliever therapy. Int J Clin Pharmacol Ther. 2015; 53:447–455. PMID:
25907171.

13. Ministry of Food and Drug Safety (KR). The Symbicort® prescribing information [Internet]. Cheongju: Ministry of Food and Drug Safety;cited 2016 Nov 8. Available from:
https://ezdrug.mfds.go.kr/.
14. Buhl R, Kuna P, Peters MJ, Andersson TL, Naya IP, Peterson S, et al. The effect of budesonide/formoterol maintenance and reliever therapy on the risk of severe asthma exacerbations following episodes of high reliever use: an exploratory analysis of two randomised, controlled studies with comparisons to standard therapy. Respir Res. 2012; 13:59. PMID:
22816878.

15. Vogelmeier C, D'Urzo A, Pauwels R, Merino JM, Jaspal M, Boutet S, et al. Budesonide/formoterol maintenance and reliever therapy: an effective asthma treatment option? Eur Respir J. 2005; 26:819–828. PMID:
16264042.
16. Søes-Petersen U, Kava T, Dahle R, Lei Y, Dam N. Budesonide/formoterol maintenance and reliever therapy versus conventional best standard treatment in asthma in an attempted ‘real life’ setting. Clin Respir J. 2011; 5:173–182. PMID:
21679353.

17. Zhong N, Lin J, Mehta P, Ngamjanyaporn P, Wu TC, Yunus F. Real-life effectiveness of budesonide/formoterol maintenance and reliever therapy in asthma patients across Asia: SMARTASIA study. BMC Pulm Med. 2013; 13:22. PMID:
23557023.

18. Bae YJ, Kim TB, Jee YK, Park HW, Chang YS, Cho SH, et al. Severe asthma patients in Korea overestimate their adherence to inhaled corticosteroids. J Asthma. 2009; 46:591–595. PMID:
19657900.

Go to :











 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download