INTRODUCTION
Intralymphatic immunotherapy (ILIT) was introduced recently as a new modality of allergen-specific immunotherapy (AIT); in this approach, only three intralymphatic injections induce marked clinical improvements as early as 4 months after the day of the first injection, and last for 3 years.
123456 Moreover, ILIT causes fewer and milder adverse reactions. However, clinical efficacy has been questioned.
7 The efficacy and adverse effects of ILIT for
Dermatophagoides farinae (Df),
Dermatophagoides pteronyssinus (Dp), and dog allergens, which are indoor allergens common globally, should be investigated. Furthermore, use of multiple allergens in ILIT warrants further investigation.
In this study, we evaluated the clinical efficacy and adverse effects of ILIT using aqueous Df, Dp, dog and cat allergens or mixtures thereof in patients with allergic rhinitis.
MATERIALS AND METHODS
Study population
We enrolled subjects with AR, symptoms of which were provoked by Df, Dp, dog, and/or cat allergens. The subjects met the following enrollment criteria described below. 1) Sensitization proven by skin prick test (SPT) and serum level of allergen-specific IgE measured by ImmunoCAP® (ThermoFisher Scientific, Uppsala, Sweden). Subjects were regarded as being sensitized to an allergen if, according to the SPT, the allergen/histamine (A/H) ratio in the wheal was ≥1 and serum level of allergen-specific IgE was ≥0.35 kU/L. 2) Complaints of AR symptoms during exposure to house dust, dog and/or cat in daily life.
Study design
At the first visit, patient eligibility was determined, information about the study was provided to subjects, written consent was obtained from subjects, and rescue medications including oral antihistamine (cetirizine) and nasal glucocorticosteroid spray (ciclesonide) were prescribed (
Supplementary Fig. 1). The patients were also asked to administer oral antihistamines with or without a nasal glucocorticosteroid spray, according to the recommendation of the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines.
8
At the second visit, pretreatment status was evaluated using questionnaires addressing allergic symptoms, and SPT, intradermal test (IDT), and nasal allergen provocation test (NAPT) results. Questionnaires used to assess allergic symptoms included the Sinonasal Outcome Test-20 (SNOT-20),
9 and Rhinoconjunctivitis Quality of life Questionnaire (RQLQ).
10 SPTs and IDTs were performed using serial dilutions of extracts of the sensitized allergen Df, Dp, dog, and/or cat (HollisterStier, New Orleans, LA, USA) according to the manufacturer's instructions. In subjects with Df and/or Dp allergy, NAPTs with the sensitized allergen were performed as previously described.
11 In addition to AR symptoms during the NAPT, the mean volume (cm
3) of the nasal cavity in the anterior nasal segment (volume 2-6 cm) was measured before the NAPT (baseline test) and every 15 minutes during NAPT by acoustic rhinometry (SRE 2000 Rhinometer; Rhinometrics, Lynge, Denmark) according to the guidelines of the Standardization Committee on Acoustic Rhinometry.
12 Subjects were asked to stop oral cetirizine (half-life: 8 h) and nasal ciclesonide spray (half-life: 3.5 h) 3 days before the second visit to ensure the validity of the SPT, IDT, and NAPT results.
At visits 3 to 5, the study subjects received three 0.1 mL injections of their sensitized allergen extract at 4-week intervals. Using ultrasound guidance and a 25-gauge needle, aqueous allergen extracts (HollisterStier, New Orleans, USA) were aseptically injected into the superficial inguinal lymph node in the right-side groin.
1234567 Before the injections, aspiration was performed to avoid inadvertent intravascular administration. After each injection, subjects were closely monitored for 1 h with checking of vital signs at 5-min intervals, and adverse events, if any, were recorded. Adverse events due to previous injections were also checked before the next injection at visits 4 and 5. Large local reactions (swelling > 10 cm in diameter that persisted for > 24 h) were identified, and systemic hypersensitivity reactions were graded using the Mueller classification.
113 At visit 3, venous puncture was performed to measure the serum levels of total IgE, allergen-specific IgE, and allergen-specific immunoglobulin G4 (IgG4), and subjects were asked to rate the pain provoked by intralymphatic injection and that by venous puncture using the visual analog scale (VAS) ranging from 0 to 100 mm.
13 The initial dose of allergen was a 1,000-fold dilution of the maximal concentration of allergen extract for subcutaneous immunotherapy (SCIT) (initial concentration: 30 AU/mL for Df or Dp; 10 AU/mL for cat hair; and 1:1/10 weight/volume (w/v) for dog hair/dander; HollisterStier) in a volume of 0.1 mL. After the first injection, the allergen concentration was escalated 3-fold on the day of the second injection, and 10-fold on the day of the third injection, if there was no or mild local or systemic hypersensitivity reaction. The allergen concentration did not change on the day of second or third injection if there was a moderate local or systemic reaction. The allergen concentration was decreased by 3- to 1,000-fold from the previous concentration if there was a severe local or systemic reaction. When 2 or more allergens were injected into the inguinal lymph node, the allergen mixture was produced in a volume of 0.1 mL to preserve the target concentration of each allergen.
At visits 6 and 7, posttreatment status was evaluated in a manner similar to that at the first visit and blood sampling was performed, respectively. Adverse events after the third injections were also checked at visit 6.
The study was approved by our Institutional Review Board and monitored by our Human Research Protection Committee. This study was registered in an open-access trials registry (ClinicalTrials.gov identifier: NCT02301884).
Statistical analysis
Statistical analysis was performed using PASW 20.0 (SPSS Inc. Chicago, IL, USA). Continuous variables were analyzed by paired Man-Whitney U test, whereas categorical variables were analyzed by Fisher's exact test. A P value <0.05 was deemed to indicate statistical significance.
RESULTS
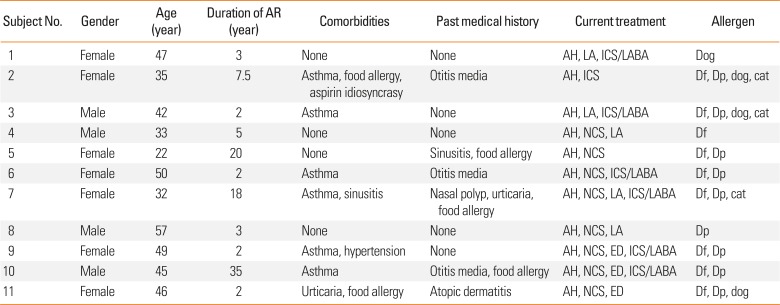
Informed consent was obtained from a total of 24 subjects; however, 4 did not attend a further visit (
Supplementary Fig. 2). Therefore, pre-ILIT evaluation of 20 subjects was carried out, but 5 dropped out due to lack of time to participate in this study, and 2 due to occurrence of anaphylaxis during SPT and IDT with Df and Dp allergens, And 2 due to lack of time after the first injection of ILIT. The post-ILIT evaluation was thus performed in 11 subjects. The demographic characteristics of the subjects are shown in
Table 1.
Table 1
Demographic characteristics
|
Subject No. |
Gender |
Age (year) |
Duration of AR (year) |
Comorbidities |
Past medical history |
Current treatment |
Allergen |
|
1 |
Female |
47 |
3 |
None |
None |
AH, LA, ICS/LABA |
Dog |
|
2 |
Female |
35 |
7.5 |
Asthma, food allergy, aspirin idiosyncrasy |
Otitis media |
AH, ICS |
Df, Dp, dog, cat |
|
3 |
Male |
42 |
2 |
Asthma |
None |
AH, LA, ICS/LABA |
Df, Dp, dog, cat |
|
4 |
Male |
33 |
5 |
None |
None |
AH, NCS, LA |
Df |
|
5 |
Female |
22 |
20 |
None |
Sinusitis, food allergy |
AH, NCS |
Df, Dp |
|
6 |
Female |
50 |
2 |
Asthma |
Otitis media |
AH, NCS, ICS/LABA |
Df, Dp |
|
7 |
Female |
32 |
18 |
Asthma, sinusitis |
Nasal polyp, urticaria, food allergy |
AH, NCS, LA, ICS/LABA |
Df, Dp, cat |
|
8 |
Male |
57 |
3 |
None |
None |
AH, NCS, LA |
Dp |
|
9 |
Female |
49 |
2 |
Asthma, hypertension |
None |
AH, NCS, ED, ICS/LABA |
Df, Dp |
|
10 |
Male |
45 |
35 |
Asthma |
Otitis media, food allergy |
AH, NCS, ED, ICS/LABA |
Df, Dp |
|
11 |
Female |
46 |
2 |
Urticaria, food allergy |
Atopic dermatitis |
AH, NCS, ED |
Df, Dp, dog |

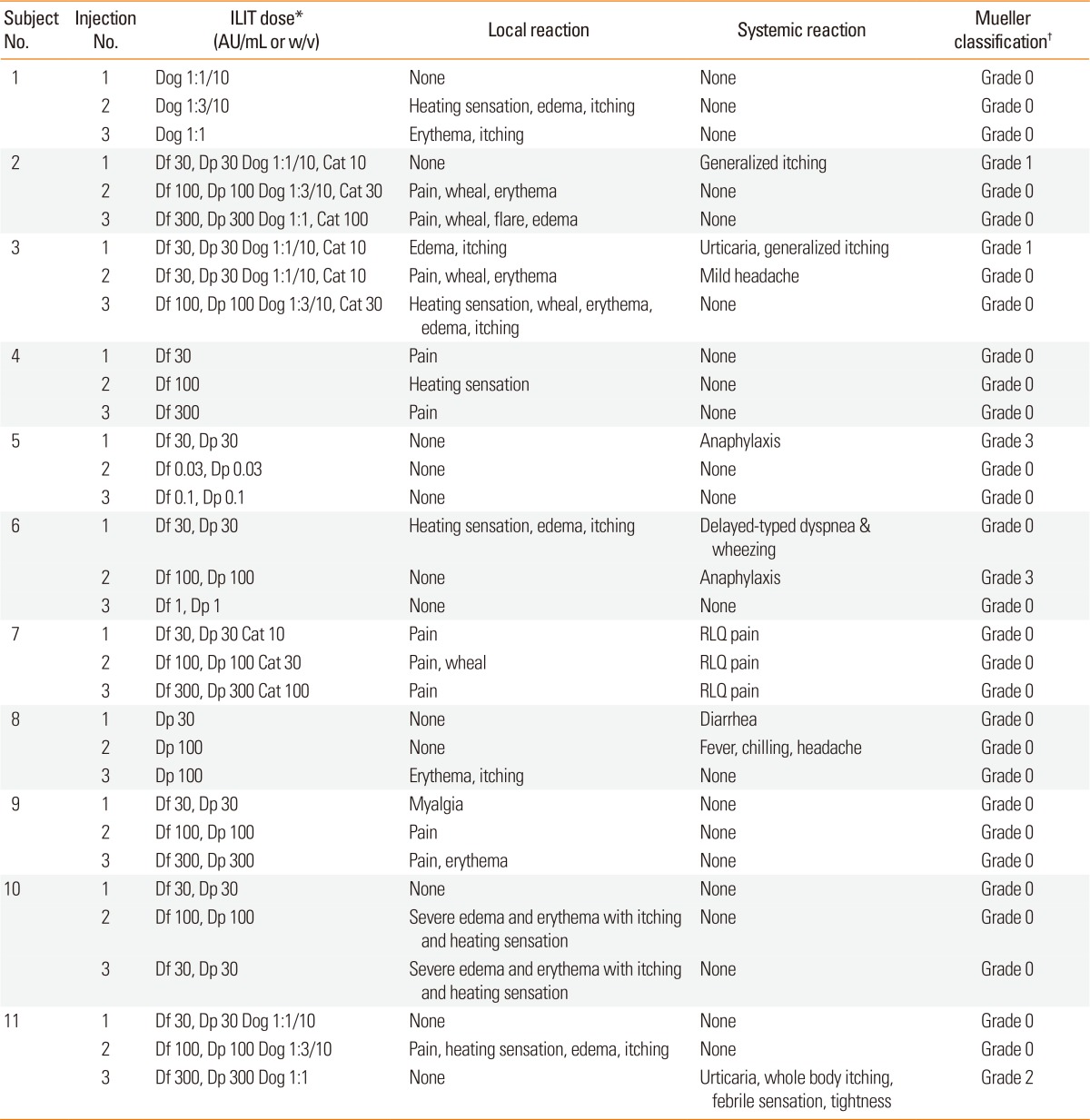
Local and systemic adverse effects during ILIT
The pain of intralymphatic injection was comparable to that of venous puncture (
Supplementary Fig. 3). Seven subjects complained of mild local or systemic reaction (grade 0-1 in the Mueller classification); however, four experienced large local reactions or moderate-to-severe systemic reactions of grade 2-3 in the Mueller classification (
Table 2). Despite those severe reactions, they strongly desired to undergo further ILIT as scheduled, so that additional injections were performed using 3-, 100-, or 1,000-fold dilutions of the concentration previously applied.
Table 2
Treatment schedule and local/systemic reactions

AR symptoms, quality of life, and prescription of rescue medication
The SNOT-20 and RQLQ scores were significantly decreased 4 months (
P=0.012 and
P=0.007, respectively) and 1 year (
P=0.047 and
P=0.009, respectively) after ILIT compared with the baseline levels (
Figure).
Figure
Subject-reported rhinitis symptoms and quality of life. (A) Sinonasal Outcome Test-20 (SNOT-20) scores. (B) Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) scores.


In general, rescue medications, with the exception of antihistamine eye drops, were prescribed less frequently after ILIT. The frequency of nasal corticosteroid spray prescription was significantly reduced 4 months after the first injection of ILIT (P=0.04; data not shown).
Nasal reactivity to house dust mites in NAPTs
Nasal symptoms during nasal challenge with house dust mite allergens in NAPTs were significantly reduced 1 year after ILIT (
P<0.05;
Supplementary Fig. 4). The decrease in nasal cavity volume during NAPTs was also alleviated 1 year after ILIT; however, the magnitude of the decrease was not significant.
Skin reactivity to allergens in the SPT and IDT
Skin reactivity to allergens in the SPT and IDT was generally increased after ILIT, albeit without statistical significance. Skin reactivity to Dp in the SPT was significantly increased 1 year after ILIT (P<0.05; data not shown).
Serum total IgE and serum allergen-specific IgE and IgG4
Serum levels of allergen-specific IgE to Df and Dp were significantly increased 4 months after ILIT (
P<0.05), but they decreased 1 year after ILIT (
P<0.05;
Supplementary Fig. 5). The serum level of allergen-specific IgG4 to Df showed a similar trend; however, it was not significantly different 4 months and 1 year after ILIT compared with baseline. The serum level of allergen specific IgG4 to Dp was significantly increased 1 year after ILIT compared with baseline (
P<0.05). Neither the serum level of allergen-specific IgE and IgG4 to dog and cat nor the serum total IgE level changed significantly after ILIT (data not shown).
DISCUSSION
All previous studies of ILIT have reported that ILIT causes only mild adverse effects.
1234567 However, we observed that ILIT could cause severe adverse reactions even at very low concentrations that were not expected to cause serious reactions in SCIT. We therefore propose that AIT of the lymph nodes is not entirely safe, as they are connected to the systemic circulation through the thoracic duct, and ILIT can cause severe adverse reactions even when very low doses of allergen are used.
In subjects who showed moderate-to-severe systemic reactions (subjects 5, 6, and 11), ILIT using Df and Dp at concentrations that, according to SPTs, led to A/H ratios in wheals of more than 1 caused systemic reactions (
Supplementary Table 1). We thus suggest that the allergen concentration be reduced in hypersensitized patients, as recommended by the manufacturer of SCIT. In detail, we propose that SPTs be performed with serial dilutions of allergens, that the initial dose of allergen in ILIT not exceed the maximal concentration leading to an A/H ratio in wheals of less than 1, and that we carefully monitor patients undergoing ILIT with allergens at doses exceeding this concentration.
No severe reaction occurred in 2 patients (subject numbers 1 and 7 in
Supplementary Table 1), although the allergen dose used in ILIT exceeded the above-mentioned concentration. Regarding severe local and systemic reactions to ILIT, we must also consider other factors such as the type and preparation of allergen and patient clinical characteristics other than hypersensitization.
Like most previous studies of ILIT, the symptoms of AR and quality of life in this study were improved as early as 4 months after the first injection of ILIT, and lasted for 1 year.
123456 In NAPTs, nasal reactivity to HDM allergens was decreased after ILIT as previously described.
12356 Furthermore, the serum levels of allergen-specific IgE and IgG
4 to Df and Dp were increased 4 months after ILIT, being consistent with the results of previous studies.
2314 However, these levels were again decreased 1 year after ILIT. Previous studies have reported decreased allergen-specific IgE levels 3 years after ILIT
1 and decreased allergen-specific IgG
4 levels 1 year after ILIT.
14 Regarding dog and cat allergens, we failed to observe any significant change in the level of allergen-specific IgE or IgG
4, due to an inadequate number of subjects. Unlike previous reports,
1267 skin reactivity to allergens in SPT and IDT generally increased after ILIT in this study.
This study has several limitations. First, it is not placebo-controlled. Therefore, the effects of other factors—including pharmacotherapy, allergen avoidance or other lifestyle modifications, natural course, and subject expectations—should be considered. Additionally, application of ILIT using multiple allergens might have hampered interpretation of the results of this study.
Despite these limitations, our study provides useful information on ILIT. First, the findings suggest that ILIT can provoke serious local or systemic reactions and that a reduced allergen dose, especially of aqueous allergen extracts, should be applied in hypersensitized patients. Secondly, this study is the first of ILIT to evaluate Df, Dp, and dog allergens, which are prevalent globally. Thirdly, this is to our knowledge the first study to use multiple allergens in ILIT.
In conclusion, ILIT can rapidly improve AR symptoms and reduce the frequency of prescription of rescue medication, and this effect lasts for 1 year. However, in hypersensitized patient, ILIT can also cause severe systemic and/or local hypersensitivity reactions when performed using aqueous allergen extract.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download