Abstract
Purpose
This study aimed to investigate the changes in the occurrence of rotavirus gastroenteritis (RGE) after the introduction of rotavirus vaccine and estimate rotavirus vaccine effectiveness in hospitalized children.
Methods
We compared the retrospective data of 671 patients with acute gastroenteritis (AGE) admitted to the Department of Pediatrics, Hanyang University Seoul Hospital from January 1, 2014, to December 31, 2015, with retrospective data of 1,243 patients admitted to the same institution with AGE from January 1, 2004, to December 31, 2005. The vaccine effectiveness was estimated using a case-positive control test-negative study.
Results
The proportion of RGE in AGE was significantly lower in 2014 to 2015 (9.0%, 48/531) than in 2004 to 2005 (22.7%, 282/1,243) (P<0.001). In particular, there was a significant decrease in the 6- to 11-, 12- to 23-, and 24- to 35-month-old groups (P<0.001), whose rotavirus vaccination rates were higher than the remaining age groups. The monthly distribution of patients with RGE in 2004 to 2005 was higher from November to May, peaked in January, followed by December and February. In 2014 to 2015, the monthly distribution of patients with RGE slightly peaked in January. In 2014 to 2015 study, the complete rotavirus vaccination rate was 66.0% (332/503) and incomplete vaccination rate was 6.2% (31/503). Presumed rotavirus vaccine effectiveness was 83.3% (95% confidence interval [CI], 60.5% to 92.9%) in the complete vaccination group and 27.4% (95% CI, –163.7% to 80.0%) in the incomplete group.
References
1. World Health Organization. Rotavirus vaccines: an update. Wkly Epidemiol Rec. 2009; 84:533–40.
2. World Health Organization. Estimated rotavirus deaths for children under 5 years of age: 2013, 215,000 [Internet]. Geneva: WHO;c2018. [cited 2018 Mar 14]. Available from:. http://www.who.int/immunization/monitoring_surveillance/burden/estimates/rotavirus/en/.
3. World Health Organization. Immunization coverage [Internet]. Geneva: WHO;c2018. [cited 2018 Mar 14]. Available from:. http://www.who.int/mediacentre/factsheets/fs378/en/.
4. Safadi MA, Berezin EN, Munford V, Almeida FJ, de Moraes JC, Pinheiro CF, et al. Hospital-based surveillance to evaluate the impact of rotavirus vaccination in Sao Paulo, Brazil. Pediatr Infect Dis J. 2010; 29:1019–22.
5. Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006; 354:23–33.

6. Chang HG, Smith PF, Tserenpuntsag B, Markey K, Parashar U, Morse DL. Reduction in hospitalizations for diarrhea and rotavirus infections in New York state following introduction of rotavirus vaccine. Vaccine. 2010; 28:754–8.

7. Payne DC, Staat MA, Edwards KM, Szilagyi PG, Weinberg GA, Hall CB, et al. Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US counties, 2006–2009. Clin Infect Dis. 2011; 53:245–53.

8. Braeckman T, Van Herck K, Raes M, Vergison A, Sabbe M, Van Damme P. Rotavirus vaccines in Belgium: policy and impact. Pediatr Infect Dis J. 2011; 30(1 Suppl):S21–4.
9. Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, Mercado J, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009; 301:2243–51.

10. Lanzieri TM, Costa I, Shafi FA, Cunha MH, Ortega-Barria E, Linhares AC, et al. Trends in hospitalizations from all-cause gastroenteritis in children younger than 5 years of age in Brazil before and after human rotavirus vaccine introduction, 1998–2007. Pediatr Infect Dis J. 2010; 29:673–5.

11. Yen C, Armero Guardado JA, Alberto P, Rodriguez Araujo DS, Mena C, Cuellar E, et al. Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J. 2011; 30(1 Suppl):S6–10.

12. The Korean Pediatric Society. Rotavirus vaccine, immunization guideline. 8th ed.Reston: The Korean Pediatric Society;2015. p. 226–38.
13. Choi UY, Lee SY, Ma SH, Jang YT, Kim JY, Kim HM, et al. Epidemiological changes in rotavirus gastroenteritis in children under 5 years of age after the introduction of rotavirus vaccines in Korea. Eur J Pediatr. 2013; 172:947–52.
15. Lee HS, Kim DY, Kim JA, Choi SH. The epidemiological trend of rotavirus gastroenteritis in children in a single center from 2004 to 2012: a retrospective study. Korean J Pediatr Infect Dis. 2014; 21:181–90.

16. Yoon SW. Study on nosocomial gastroenteritis in hospitalized children [master's thesis]. Seoul: Department of Medical Science, Hanyang University Graduate School;2009.
17. Tate JE, Patel MM, Cortese MM, Payne DC, Lopman BA, Yen C, et al. Use of patients with diarrhea who test negative for rotavirus as controls to estimate rotavirus vaccine effectiveness through case-control studies. Clin Infect Dis. 2016; 62(Suppl 2):S106–14.

18. Immergluck LC, Parker TC, Jain S, Laghaie E, Spandorfer P, Jerris RC, et al. Sustained effectiveness of monovalent and pentavalent rotavirus vaccines in children. J Pediatr. 2016; 172:116–20.
19. Boom JA, Tate JE, Sahni LC, Rench MA, Hull JJ, Gentsch JR, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics. 2010; 125:e199–207.

20. Leshem E, Givon-Lavi N, Tate JE, Greenberg D, Parashar UD, Dagan R. Real-world effectiveness of pentavalent rotavirus vaccine among Bedouin and Jewish children in Southern Israel. Clin Infect Dis. 2016; 62(Suppl 2):S155–60.

21. Fujii Y, Noguchi A, Miura S, Ishii H, Nakagomi T, Nakagomi O, et al. Effectiveness of rotavirus vaccines against hospitalisations in Japan. BMC Pediatr. 2017; 17:156.

22. Fukushima W, Hirota Y. Basic principles of test-negative design in evaluating influenza vaccine effectiveness. Vaccine. 2017; 35:4796–800.

23. El Khoury AC, Mast TC, Ciarlet M, Markson LE, Goveia MG. Projecting the effectiveness of RotaTeq(R) against rotavirus-related hospitalizations and deaths in six Asian countries. Hum Vaccin. 2011; 7:506–10.
24. Payne DC, Staat MA, Edwards KM, Szilagyi PG, Gentsch JR, Stockman LJ, et al. Active, population-based surveillance for severe rotavirus gastroenteritis in children in the United States. Pediatrics. 2008; 122:1235–43.

25. Bass DM. Rotaviruses, caliciviruses, and astroviruses. Kliegman RM, Nelson WE, editors. editors.Nelson textbook of pediatrics. 20th Ed.Philadelphia: Elsevier;2016. p. 1616–8.

26. Sohn TY, Lee CJ, Kim YJ, Kang MJ, Kim SH, Lee SY, et al. Clinical and epidemiological study of 1,165 hospitalized cases of rotaviral gastroenteritis before and after the introduction of rotavirus vaccine, 2006–2013. Korean J Pediatr Infect Dis. 2014; 21:174–80.

27. Suzuki H, Sakai T, Tanabe N, Okabe N. Peak rotavirus activity shifted from winter to early spring in Japan. Pediatr Infect Dis J. 2005; 24:257–60.

28. Torok TJ, Kilgore PE, Clarke MJ, Holman RC, Bresee JS, Glass RI. Visualizing geographic and temporal trends in rotavirus activity in the United States, 1991 to 1996. National Respiratory and Enteric Virus Surveillance System Collaborating Laboratories. Pediatr Infect Dis J. 1997; 16:941–6.
29. Kang JO, Kim MN, Kim J, Suh HS, Yoon Y, Jang S, et al. Epidemiologic trends of rotavirus infection in the Republic of Korea, July 1999 through June 2002. Korean J Lab Med. 2003; 23:382–7.
30. Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006; 354:11–22.

31. Dennehy PH, Vesikari T, Matson DO, Itzler RF, Dallas MJ, Goveia MG, et al. Efficacy of the pentavalent rotavirus vaccine, RotaTeq(R) (RV5), between doses of a 3-dose series and with less than 3 doses (incomplete regimen). Hum Vaccin. 2011; 7:563–8.
32. Gurgel RQ, Cuevas LE, Vieira SC, Barros VC, Fontes PB, Salustino EF, et al. Predominance of rotavirus P [4]G2 in a vaccinated population, Brazil. Emerg Infect Dis. 2007; 13:1571–3.
33. de Oliveira LH, Danovaro-Holliday MC, Matus CR, Andrus JK. Rotavirus vaccine introduction in the Americas: progress and lessons learned. Expert Rev Vaccines. 2008; 7:345–53.

34. Shim JO, Chang JY, Shin S, Moon JS, Ko JS. Changing distribution of age, clinical severity, and genotypes of rotavirus gastroenteritis in hospitalized children after the introduction of vaccination: a single center study in Seoul between 2011 and 2014. BMC Infect Dis. 2016; 16:287.

35. Choe YJ, Yang JJ, Park SK, Choi EH, Lee HJ. Comparative estimation of coverage between national immunization program vaccines and non-NIP vaccines in Korea. J Korean Med Sci. 2013; 28:1283–8.

36. Korean Centers for Disease Control and Prevention. National immunization survey in South Korea, 2013. Public Health Wkly Rep. 2014; 7:449–54.
Fig. 1.
Monthly distribution of patients with rotavirus gastro enteritis (RGE) and acute gastroenteritis (AGE).
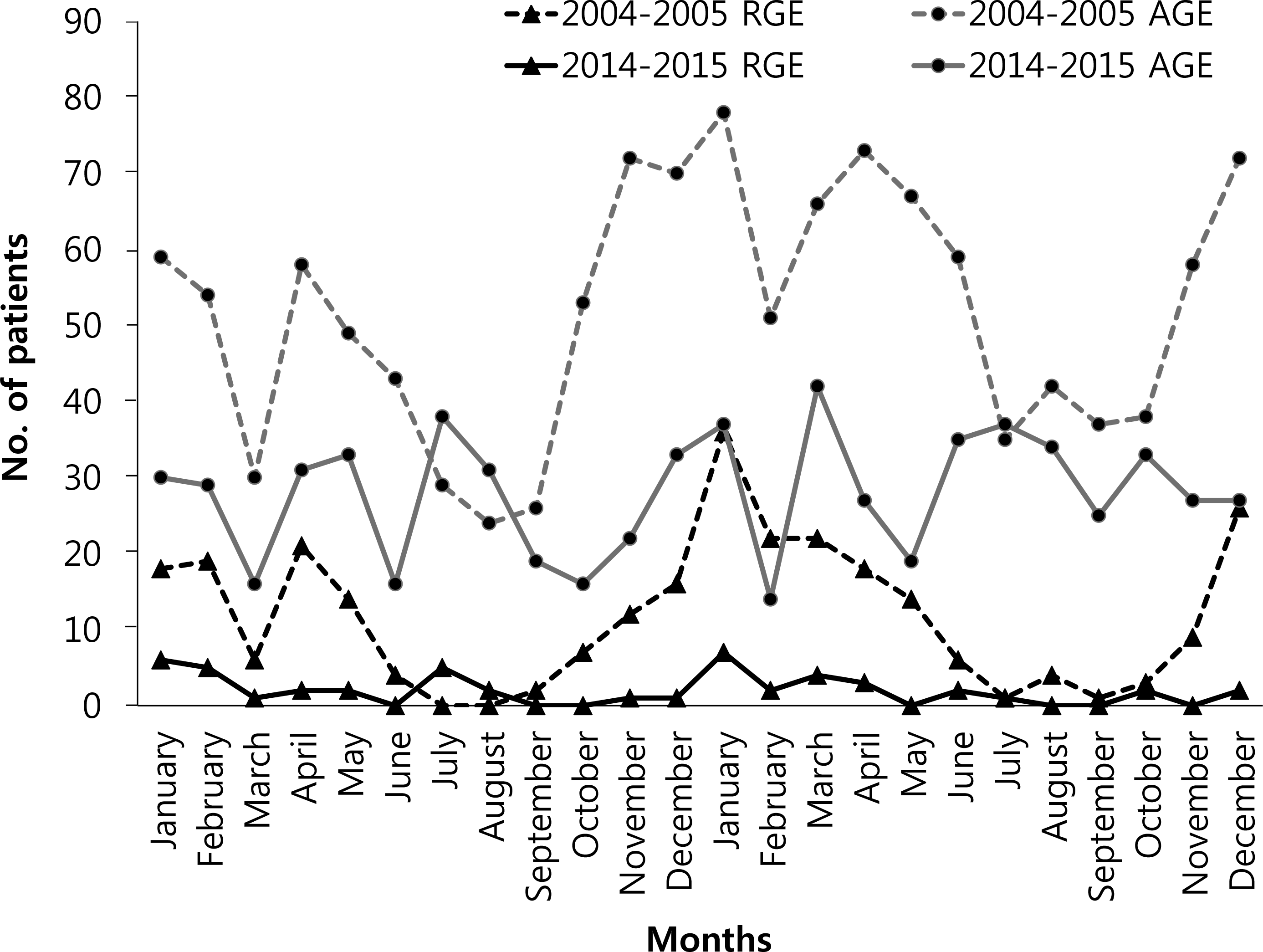
Table 1.
Age Distribution of Patients with Acute Gastroenteritis and Rotavirus Gastroenteritis
| Age group | 2004–2005 | 2014–2015 | P-value∗ | ||||
|---|---|---|---|---|---|---|---|
| AGE | RGE | RGE/AGE (%) | AGE | RGE | RGE/AGE (%) | ||
| 0–5 mo | 278 (22.4) | 39 (13.9) | 14.0 | 152 (22.7) | 20 (41.7) | 13.2 | 1.000 |
| 6–11 mo | 266 (21.4) | 48 (17.0) | 18.0 | 78 (11.6) | 0 | 0 | <0.001 |
| 12–23 mo | 322 (25.9) | 112 (39.7) | 34.8 | 127 (18.9) | 12 (25.0) | 9.4 | <0.001 |
| 24–35 mo | 142 (11.4) | 46 (16.3) | 32.4 | 67 (10.0) | 4 (8.4) | 6.0 | <0.001 |
| 36–47 mo | 89 (7.2) | 19 (6.8) | 21.3 | 42 (6.3) | 5 (10.4) | 12.0 | 0.332 |
| 48–59 mo | 68 (5.5) | 13 (4.6) | 19.1 | 35 (5.2) | 1 (2.1) | 2.9 | 1.000 |
| 60 mo–18 yr | 78 (6.3) | 5 (1.8) | 6.4 | 170 (25.3) | 6 (12.6) | 3.5 | 0.546 |
| Total | 1,243 (100) | 282 (100) | 22.7 | 671 (100) | 48 (100) | 7.2 | <0.001 |
Table 2.
Rotavirus Vaccination Status in Patients with Acute Gastroenteritis in 2014 to 2015 Study
| Vaccination status p | Rotavirus (+) patients (n=29) | Rotavirus (+) patients/ total AGE patients | P-value∗ |
|---|---|---|---|
| Received completely | 8 (27.6) | 8/332 (2.4) | <0.001 |
| Received incompletely | 3 (10.3) | 3/31 (9.7) | |
| Not received | 18 (62.1) | 18/140 (12.9) | |
| Total | 29 (100.0) | 29/503 (5.8)† |
∗ The association between the status of rotavirus vaccination and the rate of positive stool rotavirus antigen test was analyzed by the Fisher exact test.
† One hundred and sixty-eight patients with acute gastroenteritis and 19 patients with rotavirus gastroenteritis were excluded because they were not eligible for rotavirus vaccination since they were born before 2007 or under 2 months of age on study enrollment. The age of vaccinated children is between 2 and 93 months. Abbreviation: AGE, acute gastroenteritis.
Table 3.
Rotavirus Vaccination Rate by Birth Year in 2014 to 2015 Study
Table 4.
Comparison of Demographic and Clinical Characteristics of Rotavirus Gastroenteritis between Vaccinated Group and Unvaccinated Group (2014 to 2015)
| Characteristic | Vaccinated (n=11) | Unvaccinated (n=37) | P-value |
|---|---|---|---|
| Age (mo) | 23.5±23.7 | 22.7±41.7 | 0.111∗ |
| Sex ratio (male:female) | 8:3 | 23:14 | 0.723† |
| Hospital day | 4.1±2.7 | 4.1±2.0 | 0.345∗ |
| Fever | 7 (63.6) | 16 (43.2) | 0.311† |
| Duration of fever (day) | 1.3±1.6 | 0.7±0.9 | 0.228∗ |
| Vomit | 7 (63.6) | 21 (56.8) | 0.741† |
| Diarrhea | 5 (45.6) | 22 (59.5) | 0.498† |
Table 5.
Rotavirus Vaccine Effectiveness in 2014 to 2015 Study
| Vaccination status | Rotavirus-positive patients (n=29) | Rotavirus-negative control patients (n=474) | VE (95% CI) (%)∗ |
|---|---|---|---|
| Not received | 18 (62.1) | 122 (25.7) | Reference |
| Incomplete vaccination | 3 (10.3) | 28 (5.9) | 27.4 (–163.7 to 80.0) |
| Complete vaccination | 8 (27.6) | 324 (68.4) | 83.3 (60.5 to 92.9) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download