This article has been
cited by other articles in ScienceCentral.
Abstract
Severe eating disorders characterized by repetitive episodes of purging and vomiting can occasionally trigger acute kidney injury. However, interstitial nephritis induced by episodes of repeated vomiting has rarely been reported, and the pathophysiology of this entity remains unknown. A 26-year-old man was admitted to our hospital because of known hypokalemia. His serum electrolyte profile showed: sodium 133 mEq/L, potassium 2.6 mEq/L, chloride 72 mEq/L, total carbon dioxide 50 mEq/L, blood urea nitrogen/creatinine ratio (BUN/Cr) 21.9/1.98 mg/dL, and magnesium 2.0 mg/dL. Arterial blood gas analysis showed: pH 7.557, partial pressure of carbon dioxide 65.8 mmHg, and bicarbonate 58.5 mEq/L. His urinary potassium concentration was 73.2 mEq/L, and Cr was 111 mg/dL. Renal biopsy revealed acute tubular necrosis and tubulointerstitial nephritis with a few shrunken glomeruli. Repeated psychogenic vomiting may precipitate acute kidney injury and interstitial nephritis secondary to volume depletion and hypokalemia. Serum electrolyte levels and renal function should be carefully monitored in patients diagnosed with eating disorders to prevent tubular ischemia and interstitial nephritis.
Go to :

Keywords: Interstitial nephritis, anorexia, hypokalemia, tubular necrosis
Introduction
Eating disorders are common psychiatric disorders observed in young women. Most patients are observed to develop eating disorders by the age of 25 years. The incidence of eating disorders in men is 10-fold lower than that in women
1). Anorexia nervosa is an eating disorder characterized by the inability to maintain a normal body weight, a severe disturbance of body image, and cessation of the menstrual cycle. Prolonged anorexia can cause malnutrition and various medical conditions. Metabolic acidosis or alkalosis may develop in patients with severe anorexia secondary to hypochloremia induced by prolonged vomiting
2). Renal dysfunction observed in patients with eating disorders includes an abnormal urinalysis, electrolyte abnormalities (most commonly hypokalemia), and azotemia
3). Repeated episodes of vomiting cause chronic volume depletion and consequently trigger renal tubular ischemia. Vomiting-induced chronic hypokalemia and metabolic alkalosis can cause tubular deposition of calcium and protein
4). However, data describing renal biopsy findings of tubular damage caused by eating disorders are limited. We report a case of renal biopsy-proven tubulointerstitial damage caused by repeated vomiting and chronic hypokalemia.
Go to :

Case Report
A 26-year-old man was admitted with known hypokalemia. He reported a 3-year history of nausea, vomiting, and diffuse muscle weakness. He complained of nausea and epigastric fullness in the absence of any identifiable organic gastrointestinal pathology, and his symptoms were known to subside after self-induced vomiting. Although he reported a normal appetite, he was known to vomit frequently, particularly after a large meal. He had been diagnosed with hypokalemia and had been treated with potassium supplements at a previous hospital. However, because of persistent nausea and vomiting, he was transferred to our hospital for evaluation of hypokalemia. His medical and family histories were unremarkable, and he did not report the use of medications such as diuretics and/or herbal medication. Physical examination upon admission showed he was 175 cm tall and weighed 44.5 kg, which indicated a weight loss from his previous weight of 58 kg over the previous 2 years. His body mass index was 14.53 kg/m2. Blood pressure was 100/60mmHg, pulse rate was 67/min, respiratory rate was 20/min, and his body temperature was 37.0℃.
Initial serum electrolyte testing showed the following: sodium 133 mEq/L, potassium 2.6 mEq/L, chloride 72 mEq/L, and total carbon dioxide 50mEq/L. Calcium/phosphorus were 11.2/4.1mg/dL, blood urea nitrogen/creatinine were 21.9/1.98mg/dL, the estimated glomerular filtration rate was 43.6mL/min/1.73m2, serum magnesium was 2.0mg/dL, blood glucose was 93mg/dL, and hemoglobin was 12.0 g/dL. Total cholesterol was 285mg/dL, serum protein/albumin were 8.1/5.3 g/dL. Thyroid and adrenal function tests, as well as serum renin and aldosterone levels were all within reference range. Arterial blood gas analysis showed pH 7.557, partial pressure of carbon dioxide was 65.8mmHg, and bicarbonate was 58.5 mEq/L. Urinary levels of sodium/potassium/chloride (Na/K/Cl) were 95/73.2/47mEq/L, respectively, his urinary creatinine measured 111.12mg/dL, and the trans-tubular potassium gradient was 17. Urinalysis showed the following findings: blood -, albumin ±, glucose -, and ketones -. Upper endoscopy and abdominal computed tomography showed no abnormalities. The SLC12A3 gene mutation test for Gitelman syndrome was observed to be negative. He was diagnosed with chloride-responsive metabolic alkalosis with kidney failure and treated with saline hydration, potassium supplementation, and spironolactone. Serum potassium increased to 3.0mEq/L; however, his nausea and vomiting persisted.
A renal biopsy was performed for the evaluation of renal dysfunction. Microscopically, interstitial mononuclear cell infiltration was identified. Renal tubular epithelial cells showed mild nuclear variation with conspicuous nucleoli and cytoplasmic vacuolization, indicating regenerative changes after acute tubular necrosis. Several foci of dystrophic calcification were identified among the tubular epithelial cells. A few shrunken glomeruli were observed suggesting ischemic damage; however, most glomeruli revealed no definitive abnormalities such as mesangial cell proliferation, segmental sclerosis, and/or deposition of immune complexes (
Fig. 1). The patient showed an improvement in his gastrointestinal symptoms after treatment with mirtazapine. His serum potassium level increased to 3.5–4.4mEq/L and to date, he has been following-up at the outpatient clinic without any clinical signs and symptoms.
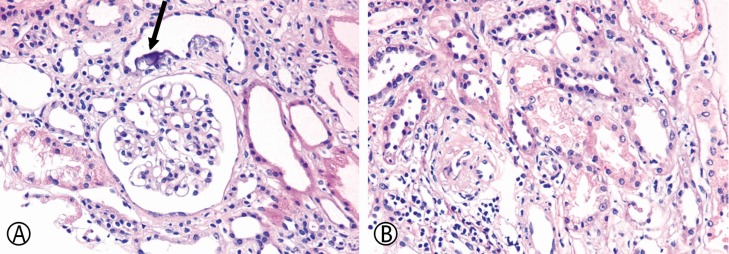 | Fig. 1(A) Renal tubules showing calcification below the epithelial cells (arrow) without definite glomerular abnormality (hematoxylin and eosin, ×400). (B) Vacuolar changes in the cytoplasm of cells of the proximal tubular epithelium. Mild nuclear variation, implying regeneration after acute damage, is also apparent (hematoxylin and eosin, ×400).
|
Go to :

Discussion
Self-induced vomiting is a common means of weight reduction observed among young women, although a few patients who use diuretics or who deliberately vomit deny doing so. In patients presenting with psychogenic vomiting, hypokalemia is the most common electrolyte abnormality observed in clinical practice, which may induce fatal arrhythmias in old age
5). Although previous reports have described an association between eating disorders and kidney disease
67), the exact pathophysiology remains unclear.
Hypokalemia is a known cause of renal injury. Early stages of hypokalemic nephropathy are usually reversible, and patients are known to recover following potassium-replacement therapy; however, persistent hypokalemia causes tubulointerstitial nephritis and irreversible renal injury
8). Prolonged potassium depletion causes characteristic vacuolar lesions within the epithelial cells of the proximal tubules and, less frequently, the distal tubules. Nonspecific glomerulosclerosis and hyperplasia of the juxtaglomerular apparatus may also occur in a few cases
27910). Chronic hypokalemia, which is a common abnormality observed in patients with eating disorders, may cause severe kidney damage, including interstitial nephritis and fibrosis, tubular atrophy, and medullary cyst formation
11). The pathogenesis of this kidney injury has not been completely understood. An increase in the renal production of ammonium, enhanced renal cytogenesis, and alterations in growth factors and cytokines are implicated as possible etiological contributors
12).
Riemenschneider et al. described the renal morphology in 40 patients who showed prolonged hypokalemia and hyponatremia, in whom an increase in the extent of the interstitial area correlated with renal function
9). In our patient, we observed nonspecific calcification of the renal tubules at sites beneath the epithelial cells, which we reckon could be attributed to sustained hypokalemia. However, usually, hypokalemia alone is not known to cause tubular necrosis and inflammation. The patient was bulimic during each meal and would induce vomiting, particularly after a large meal. We concluded that repeated episodes of vomiting caused sustained volume depletion and precipitated tubulointerstitial ischemia, although his glomeruli were relatively well-preserved. Our findings suggest that even short-term episodes of hypokalemia and anorexia nervosa, if repeated, can cause tubulointerstitial injuries in previously healthy young men. Moreover, this case report could be useful to gain a better understanding of the pathophysiology of anorexia nervosa-induced tubulointerstitial nephropathy.
Go to :

Conclusion
In conclusion, even short-term episodes of repeated psychogenic vomiting associated with only mild volume depletion may cause renal injury and interstitial fibrosis. We emphasize that serum electrolyte levels and renal function should be carefully monitored in patients with eating disorders to prevent tubular ischemia and interstitial nephritis, which may cause irreversible kidney injury.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download