Abstract
Paraganglioma in pregnancy is an extremely rare condition and its diagnosis is often delayed because the clinical symptoms can mimic those of preeclampsia or gestational hypertension. Here, we report the case of a 32-year-old, gravida 2, para 1 woman who presented with severe headache, palpitation, and sweating at 37 weeks' gestation. Although emergent cesarean section was performed on the assumption of severe preeclampsia, blood pressure fluctuated and heart rate remained tachycardiac. We suspected that she might have thromboembolic lesion in the chest or pheochromocytoma. Chest and abdominal computed tomography revealed a 4 cm mass in the left para-aortic space. Serum and urinary catecholamine levels were found to be significantly increased. She underwent laparoscopic mass removal and the pathology confirmed paraganglioma. When typical paroxysmal hypertension is accompanied by headache, palpitation, and sweating during pregnancy, adrenal tumors should be considered.
Approximately 10% of all deliveries in the United States are complicated by hypertensive disorders, with preeclampsia/eclampsia being one of the 3 major causes of maternal morbidity and mortality. Preeclampsia/eclampsia-related deaths account for more than 50,000 maternal deaths worldwide every year, occurring at a rate of 1.5/100,000 live births [12].
However, preeclampsia can be confused with many other obstetric, medical or surgical conditions, including acute fatty liver, cholestasis of pregnancy, thrombotic microangiopathy, catecholamine-secreting tumors (CSTs), disseminated herpes simplex, and septic shock, among others [3]. Because the clinical presentation and laboratory findings of these conditions are similar to those of preeclampsia, differential diagnosis can be challenging. Among these imitators of preeclampsia, CSTs in pregnancy can lead to catastrophic results if not recognized at the appropriate time. Herein, we report a case of paraganglioma masquerading as severe preeclampsia.
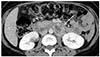
A 32-year-old Korean woman, gravida 2, para 1, was referred for uncontrolled high blood pressure (systolic blood pressure >200 mmHg) at 37 weeks' gestation. She complained of severe headache and posterior neck pain. She was otherwise asymptomatic and there was no remarkable past medical history or family history. Upon physical examination, she had no fever, but was soaked in sweat. On admission, her blood pressure was 220/137 mmHg and her heart rate was over 140 beats/minute. Her systolic blood pressure remained higher than 200 mmHg even though she was treated with hydralazine, labetalol, and magnesium sulfate. Routine laboratory findings revealed that hepatic enzyme levels were mildly elevated at 60 IU/L for aspartate aminotransferase, 50 IU/L for alanine aminotransferase, and a 4+ dipstick urine protein reaction was noted. A presumptive diagnosis of severe preeclampsia was made. At that time, regular uterine contractions were detected on cardiotocography and prolonged fetal heart rate deceleration was observed. An emergent cesarean section was immediately performed under general anesthesia. A male infant weighing 3,670 g was delivered and the Apgar score was 8/9 at 1/5 minutes. Focal placenta abruptio was found upon gross examination and histology later confirmed focal infarction and hemorrhage. During the induction of anesthesia and the operation, blood pressure fluctuated between 210/130 mmHg and 180/90 mmHg. Nicardipine and nitroglycerin were administered continuously. During recovery, her blood pressure ranged from 160/100 mmHg to 140/90 mmHg and she complained of intermittent headache, palpitation, dizziness, and excessive sweating. Tachycardia of 140–160 beats/minute failed to respond to medications. We suspected a thromboembolic lesion in the chest or possibly a pheochromocytoma. There was no evidence of pulmonary thrombosis on chest computed tomography imaging, but the patient had a 4-cm sized mass in the para-aortic space near the left renal hilum (Fig. 1). Paraganglioma was strongly suspected. Measurement of 24-hour urine catecholamines demonstrated elevated norepinephrine and metanephrine levels of 91.7 μg/day (reference range [RR], 15–80) and 5.5 mg/day (RR, 0–1.3), respectively. Her plasma normetanephrine level was elevated at 5,710 pmol/L (RR, 0–900). Echocardiography revealed normal cardiac function. Treatment with doxazosin and nifedipine was started. She remained mildly hypertensive (140/90 mmHg) to normotensive with no hyperadrenergic complicated symptoms during recovery. Additional positron emission tomography scanning was done to detect hypermetabolic tumors and their metastasis. There was one hypermetabolic mass in the left para-aortic space but no metastatic masses were found. On subsequent laparotomy, a 4-cm sized round, firm, encapsulated tumor was surgically removed. During tumor removal, the patient's blood pressure rose to 190/110 mmHg by just gentle manipulation. The patient's postoperative course was uneventful and her blood pressure and heart rate stayed within normal range without the use of antihypertensive drugs. Pathologic findings revealed classical features of paraganglioma (Fig. 2A). The resection margins were clear and focal necrosis was observed. The diagnosis was firmly established from positive immunohistochemistry staining for chromogranin A (Fig. 2B). The patient was discharged in good condition without any medication. Blood tests and urinary assays of catecholamine, performed at post-operative 4 weeks, revealed that the values had normalized.
CSTs can arise from any site along the migratory path of chromaffin cells, which are present in the adrenal medulla and scattered extramedullary paraaortic paraganglia. Originally, these extramedullary paraganglia tissues gradually disappear after birth and are gone by 2 to 3 years of age [4]. According to the 2004 World Health Organization classification, adrenal CSTs can be divided into intra-adrenal paraganglioma, which is more commonly referred to as pheochromocytoma, and extra-adrenal paraganglioma. Extra-adrenal paragangliomas can originate from either sympathetic or parasympathetic chain paraganglia. Sympathetic paraganglioma is usually a catecholamine-secreting functional tumor and is mainly located in the abdomen and thorax [5]. On the other hand, most paragangliomas deriving from the parasympathetic chain are catecholamine non-secreting and located in the neck and skull base [6]. In this case report, the term for paraganglioma was used to designate sympathetic paraganglioma.
CSTs rarely present in pregnancy and are estimated to occur in only 7 cases per 100,000 pregnancies [7]. Although the specific incidence of paraganglioma alone remains unclear, it is presumed to be less common than pheochromocytoma. A review of 77 cases of complicated pregnancies with CSTs between 2000 and 2011 revealed that 80% of cases were pheochromocytomas and 20% of cases were paragangliomas [8]. In this case, it was very fortuitous that both the mother and the infant survived, as the mortality rates for both are high in cases of paraganglioma recognized in the postpartum period. The review article mentioned above also reported that 73% of the 77 reviewed cases were diagnosed antenatally, 27% of cases were identified in the postnatal period or only after fetal and/or maternal death [8]. When the diagnosis was made in the antenatal period, the mortality rate was 12% for the fetus and 0% for the mother. In contrast, 27% of cases undiagnosed during pregnancy resulted in 29% mortality rate for both the fetus and mother respectively [8]. The vasoconstrictive effects of catecholamines may impair uteroplacental perfusion, leading to fetal hypoxia with growth retardation, placental abruptio, and even fetal demise [9]. Our patient's infant might have suffered from a hypoxic event resulting in late deceleration antenatally and placenta abruptio.
Despite its high mortality rate, masquerading symptoms that mimic preeclampsia or gestational hypertension can delay accurate diagnosis [10]. This patient presented with confusing clinical symptoms and laboratory findings in which a paraganglioma pretended to be mimicking severe preeclampsia. Severe hypertension accompanied by proteinuria and abnormal liver function tests supported the diagnosis of severe preeclampsia, although sustained tachycardia and hyperhidrosis are not typical signs. Moreover, preeclampsia usually occurs after 20 weeks of gestation, compared to CSTs which can occur during any gestational phase [11]. The fact that this patient only had her first paroxysmal hypertensive attack in the third trimester led us to exclude the possibility of paraganglioma or pheochromocytoma. However, a previously silent tumor can manifest symptoms for the first time at late gestational age due to increased abdominal pressure related to the enlarged uterus, fetal movements, uterine contractions, physical or emotional stress, labor, and spontaneous hemorrhage of the tumor itself [9]. Consequently, physicians should be aware of the classic triad symptoms of sweating, palpitation, and headache associated with these tumors at any gestational age. Furthermore, this case was presented to emphasize that paraganglioma or pheochromocytoma should be considered in the differential diagnosis of peripartum tachycardia, especially when associated with paroxysmal hypertension.
Measurement of increased catecholamine and its metabolites on urinary and plasma tests is required to confirm the diagnosis. Paragangliomas almost always produce norepinephrine such that its metabolite, normetanephrine, is overproduced [12]. In our case, plasma normetanephrine, urinary norepinephrine and urinary metanephrine levels were elevated. Although elevated plasma or urinary catecholamine levels were reported in severe preeclampsia cases, these are rare conditions [13].
Conventional first line antihypertensive treatment for severe preeclampsia includes direct vasodilators, combined alpha-beta blockers, and calcium channel blockers. Although her labile blood pressure was relatively well controlled by these multi-drug regimens, her heart rate did not respond to these medications. After the diagnosis was made, an alpha 1-adrenoceptor antagonist and a calcium channel blocker were used together, resulting in stable heart rates. Using beta blockers, especially non-selective ones, before sufficient suppression of alpha adrenoceptor can cause hypertensive crises [14]. The combined alpha-beta adrenoceptor blocker that we initially used on the admission day may also lead to paradoxical hypertensive responses [15].
In conclusion, early suspicion and differential diagnosis of paraganglioma and pheochromocytoma in pregnancy are important to reduce adverse obstetric outcomes. Physicians who care for sporadic and untractable hypertension in pregnant women should be aware of the symptoms associated with these tumors.
References
1. Dhariwal NK, Lynde GC. Update in the management of patients with preeclampsia. Anesthesiol Clin. 2017; 35:95–106.

2. Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. Br J Obstet Gynaecol. 1992; 99:547–553.

4. DeGroot LJ, Jameson JL. Endocrinology. 5th ed. Philadelphia (PA): Elsevier Saunders;2006.
5. Erickson D, Kudva YC, Ebersold MJ, Thompson GB, Grant CS, van Heerden JA, et al. Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab. 2001; 86:5210–5216.

6. Snabboon T, Plengpanich W, Houngngam N, Buranasupkajorn P, Plengvidhya N, Sereepapong W, et al. Concurrent bilateral pheochromocytoma and thoracic paraganglioma during pregnancy. Endocrine. 2010; 37:261–264.

7. Harrington JL, Farley DR, van Heerden JA, Ramin KD. Adrenal tumors and pregnancy. World J Surg. 1999; 23:182–186.

8. Biggar MA, Lennard TW. Systematic review of phaeochromocytoma in pregnancy. Br J Surg. 2013; 100:182–190.

9. Lenders JW. Pheochromocytoma and pregnancy: a deceptive connection. Eur J Endocrinol. 2012; 166:143–150.
10. Lyman DJ. Paroxysmal hypertension, pheochromocytoma, and pregnancy. J Am Board Fam Pract. 2002; 15:153–158.
11. Prete A, Paragliola RM, Salvatori R, Corsello SM. Management of catecholamine-secreting tumors in pregnancy: a review. Endocr Pract. 2016; 22:357–370.

12. Al-Harthy M, Al-Harthy S, Al-Otieschan A, Velagapudi S, Alzahrani AS. Comparison of pheochromocytomas and abdominal and pelvic paragangliomas with head and neck paragangliomas. Endocr Pract. 2009; 15:194–202.

13. Øian P, Kjeldsen SE, Eide I, Maltau JM. Increased arterial catecholamines in pre-eclampsia. Acta Obstet Gynecol Scand. 1986; 65:613–617.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download