1. Norris HJ, Parmley T. Mesenchymal tumors of the uterus. V. Intravenous leiomyomatosis. A clinical and pathologic study of 14 cases. Cancer. 1975; 36:2164–2178.

2. Nam MS, Jeon MJ, Kim YT, Kim JW, Park KH, Hong YS. Pelvic leiomyomatosis with intracaval and intracardiac extension: a case report and review of the literature. Gynecol Oncol. 2003; 89:175–180.

3. Solomon LA, Schimp VL, Ali-Fehmi R, Diamond MP, Munkarah AR. Clinical update of smooth muscle tumors of the uterus. J Minim Invasive Gynecol. 2005; 12:401–408.

4. Clement PB, Young RH, Scully RE. Intravenous leiomyomatosis of the uterus. A clinicopathological analysis of 16 cases with unusual histologic features. Am J Surg Pathol. 1988; 12:932–945.
5. Vaquero ME, Magrina JF, Leslie KO. Uterine smooth-muscle tumors with unusual growth patterns. J Minim Invasive Gynecol. 2009; 16:263–268.

6. Patton KT, Cheng L, Papavero V, Blum MG, Yeldandi AV, Adley BP, et al. Benign metastasizing leiomyoma: clonality, telomere length and clinicopathologic analysis. Mod Pathol. 2006; 19:130–140.

7. Miller J, Shoni M, Siegert C, Lebenthal A, Godleski J, McNamee C. Benign metastasizing leiomyomas to the lungs: an institutional case series and a review of the recent literature. Ann Thorac Surg. 2016; 101:253–258.

8. Lee HJ, Choi J, Kim KR. Pulmonary benign metastasizing leiomyoma associated with intravenous leiomyomatosis of the uterus: clinical behavior and genomic changes supporting a transportation theory. Int J Gynecol Pathol. 2008; 27:340–345.

9. Lewis EI, Chason RJ, DeCherney AH, Armstrong A, Elkas J, Venkatesan AM. Novel hormone treatment of benign metastasizing leiomyoma: an analysis of five cases and literature review. Fertil Steril. 2013; 99:2017–2024.

10. Ma G, Miao Q, Liu X, Zhang C, Liu J, Zheng Y, et al. Different surgical strategies of patients with intravenous leiomyomatosis. Medicine (Baltimore). 2016; 95:e4902.

11. Ordulu Z, Nucci MR, Dal Cin P, Hollowell ML, Otis CN, Hornick JL, et al. Intravenous leiomyomatosis: an unusual intermediate between benign and malignant uterine smooth muscle tumors. Mod Pathol. 2016; 29:500–510.

12. Worley MJ Jr, Aelion A, Caputo TA, Kent KC, Salemi A, Krieger KH, et al. Intravenous leiomyomatosis with intracardiac extension: a single-institution experience. Am J Obstet Gynecol. 2009; 201:574.e1–574.e5.

13. Cummings M, Merone L, Keeble C, Burland L, Grzelinski M, Sutton K, et al. Preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios predict endometrial cancer survival. Br J Cancer. 2015; 113:311–320.

14. Yu X, Zhang G, Lang J, Liu B, Zhao D. Factors associated with recurrence after surgical resection in women with intravenous leiomyomatosis. Obstet Gynecol. 2016; 128:1018–1024.

15. Carr RJ, Hui P, Buza N. Intravenous leiomyomatosis revisited: an experience of 14 cases at a single medical center. Int J Gynecol Pathol. 2015; 34:169–176.
16. Canzonieri V, D'Amore ES, Bartoloni G, Piazza M, Blandamura S, Carbone A. Leiomyomatosis with vascular invasion. A unified pathogenesis regarding leiomyoma with vascular microinvasion, benign metastasizing leiomyoma and intravenous leiomyomatosis. Virchows Arch. 1994; 425:541–545.

17. Bodner-Adler B, Bartl M, Wagner G. Intravenous leiomyomatosis of the uterus with pulmonary metastases or a case with benign metastasizing leiomyoma? Anticancer Res. 2009; 29:495–496.



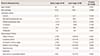
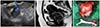
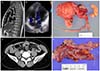




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download