Introduction
Cervical cancer can often be prevented through screening and treatment of precursor lesions. Diagnosis and management of cervical intraepithelial neoplasia (CIN) are critical steps in cancer prevention. Currently, the treatment techniques of CIN require that patients with high-grade cervical punch biopsy results should undergo ablation or conization. Although usually well tolerated, loop electrosurgical excision procedure (LEEP) has risks, such as bleeding, infection, and reproductive complications. Therefore, the indication whether LEEP should be performed is important. Identifying the significant factors that influence the pathologic discrepancy between the punch biopsy and LEEP conization may be an important step in preventing unnecessary LEEP.
According to Duesing et al. [
1], the overall sensitivity of biopsy and cone pathology is 50–70% and 55–90% for CIN1 and CIN2/3, respectively, while specificity varies from 80% for CIN1 to 96% for CIN2/3. In cases of patients with high-grade cervical punch biopsy results, 14–24% of the cone histopathology showed low-grade lesions [
2345]. Ryu et al. [
6] shows only low human papillomavirus (HPV) load (cutoff=100 RLU) without age, Pap test, punch biopsy grade, and HPV genotype as the predictive factor in biopsy overestimation. Rodriguez-Manfredi et al. [
4] also represents that low viral load and negative HPV are influencing factors in biopsy overestimation. Kjellberg [
7] recommended that high-grade squamous intraepithelial lesion (HSIL) can be followed immediately by colposcopically guided LEEP without punch biopsy.
The reported concordance rate between colposcopically directed punch biopsy and cone histopathology was 42–57% [
89]. In addition, some studies reported that punch biopsies are accurate, whereas others report false-negative rates of up to 50% [
10]. The discrepancy of concordance between colposcopically directed punch biopsy and subsequent histopathological conization finding is common and present a unique clinical challenge. Limited accuracy of colposcopically directed punch biopsy and inconsistency of pathology with LEEP conization remain an important clinical problem. Biopsy results that underestimate the CIN grade (biopsy underestimation) may have serious implications if conization was not performed. Although LEEP conization has several advantages, such as being simple, safe, and inexpensive, biopsy overestimation is also dangerous because adverse effects on fertility and pregnancy outcomes by unnecessary conization may occur. We identified that many clinical factors can be hypothesized to influence the concordance of the 2 steps. In the present study, we evaluated the major clinical factors that affect the inaccuracy of CIN diagnosis. The purpose of our study was to investigate the factors affecting discrepancy, particularly in reproductive age women, reducing unnecessary conization, and biopsy overestimation, and help to establish appropriate treatment and follow-up plans for the patients.
Go to :

Materials and methods
This study was approved by the Institutional Review Board of Daejeon St. Mary's Hospital at the Catholic University of Korea. Between 2004 and 2016, 1,200 patients who underwent LEEP conization were identified in the gynecologic oncology department of Daejeon St. Mary's Hospital. Of these, 667 patients who underwent both cervical cytology, HPV typing, colposcopically punch biopsy, and LEEP conization were included in this retrospective study. All the patients who participated in the study was performed liquid-based Pap (LBP) test and HPV DNA typing. For HPV DNA typing, HPV DNA chip was used. Only patients with known results of Pap test, HPV DNA test, and those who underwent colposcopically punch biopsy and LEEP conization in our hospital were enrolled in our study. None of the included women were pregnant and patients with glandular abnormalities or previous conization were excluded. All procedures of the next step were performed when the previous steps showed abnormal results. The colposcopic experience of the physicians was between 5 and 10 years for 4 physicians and more than 10 years for 1 physician. During the study period, the institutional approach in case of cytologically suspected dysplasia consisted of a work-up with colposcopy, HPV DNA typing and punch biopsy. LEEP was performed in case of a CIN2/3 detection in punch biopsy or as a diagnostic procedure in case of highly suspected CIN2/3 in colposcopy and cytology despite insignificant biopsy or in case of an invisible transformation zone. Also, in case of micro invasive carcinoma (MIC) or inaccurate depth of invasion from punch biopsy, we performed conization for initial therapy of MIC and decision for extent of next treatment in cancer. Histopathologic examinations of punch biopsy and LEEP conization were analyzed by the gynecopathologist at our hospital. The slides for all the cervical cones as well as the presurgical LBP test and biopsy specimens were reviewed by a panel of 3 pathologists (H.J.S., J.O.K., and L.I.L.). All biopsy specimens were reviewed by at least 2 panel. The histopathologic results of either biopsy or conization can bed is cordant to each other. Biopsy underestimation was defined as detecting more severe lesions by conization when compared to biopsies and biopsy overestimation was defined as detecting less severe lesions diagnosed by conization when compared to biopsies.
All data were collected by chart review, and the cytologic classification used was the 2014 Bethesda System terminology, and histopathologic findings were classified based on the American Society for Colposcopy and Cervical Pathology (ASCCP).
Variable factors from patients were recorded for indication for conization, age, menopausal status, number of delivery and abortion, Pap test, visualization of the entire transformation zone, number of punch biopsy, HPV type, time interval between punch biopsy and LEEP, and histological diagnosis of punch biopsy and LEEP conization.
Analysis of data was performed with SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) by using descriptive statistical methods. The correlation between histopathologic findings of punch biopsy and conization was assessed by kappa statistics (κ). We used univariable and multivariable analyses to evaluate the association between clinical variables and the diagnosis. Univariable logistic regression analysis was used to test all studied independent variables and the results were expressed as odds ratio (OR) with 95% confidence intervals (CIs). Multivariable logistic regression analysis was used to identify independent variables that associated significantly with final diagnosis and discrepancy of punch biopsy and conization. A P-value of less than 0.05 was considered to indicate statistical significance.
Go to :

Results
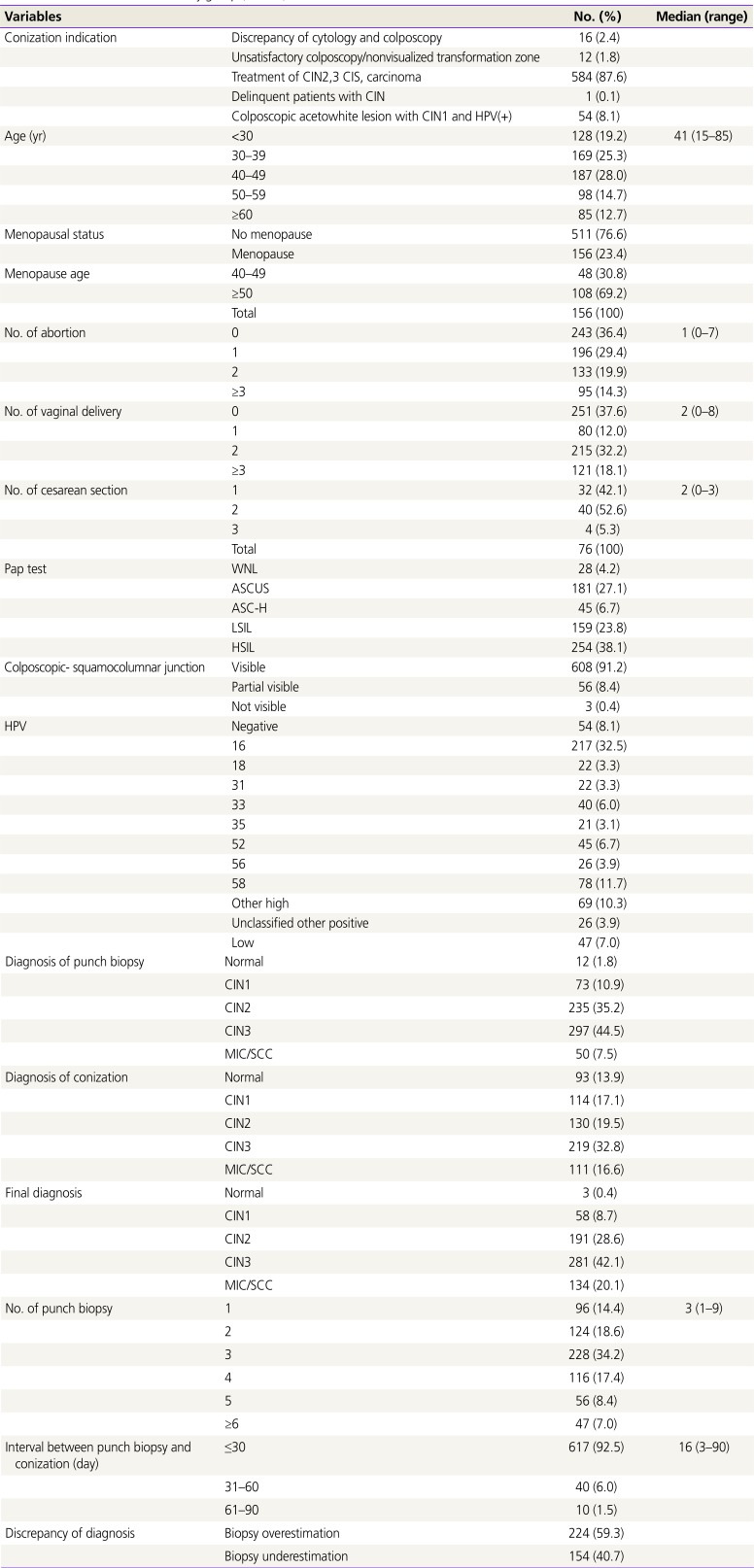
Table 1 shows the characteristics of the study group. The median age of the 667 patients was 41 years (range, 15–85 years), and 511 and 156 were premenopausal and menopausal patients, respectively. The median number of abortion, vaginal delivery, and cesarean delivery was 1.0, 2.0, and 2.0, respectively. The colposcopic squamocolumnar junction, duration between punch and conization and number of punch biopsies are shown in
Table 1. The colposcopic findings were divided into visible, partially visible, and non-visible based on the degree to which the squamocolumnar junction was shown. In the cytology, HSIL, atypical squamous cells of undetermined significance (ASCUS) were 38.1% and 27.1%, respectively, and punch biopsy and conization showed high incidence of CIN3 at 44.5% and 32.8%, respectively. In HPV, the high type group with frequency of <3% was classified as other high type groups, and other high type groups included 39, 45, 51, 53, 59, 66, 68 and 73.
Table 1
Characteristics of the study group (n=667)
|
Variables |
No. (%) |
Median (range) |
|
Conization indication |
Discrepancy of cytology and colposcopy |
16 (2.4) |
|
|
Unsatisfactory colposcopy/nonvisualized transformation zone |
12 (1.8) |
|
Treatment of CIN2,3 CIS, carcinoma |
584 (87.6) |
|
Delinquent patients with CIN |
1 (0.1) |
|
Colposcopic acetowhite lesion with CIN1 and HPV(+) |
54 (8.1) |
|
Age (yr) |
<30 |
128 (19.2) |
41 (15–85) |
|
30–39 |
169 (25.3) |
|
40–49 |
187 (28.0) |
|
50–59 |
98 (14.7) |
|
≥60 |
85 (12.7) |
|
Menopausal status |
No menopause |
511 (76.6) |
|
|
Menopause |
156 (23.4) |
|
Menopause age |
40–49 |
48 (30.8) |
|
|
≥50 |
108 (69.2) |
|
Total |
156 (100) |
|
No. of abortion |
0 |
243 (36.4) |
1 (0–7) |
|
1 |
196 (29.4) |
|
2 |
133 (19.9) |
|
≥3 |
95 (14.3) |
|
No. of vaginal delivery |
0 |
251 (37.6) |
2 (0–8) |
|
1 |
80 (12.0) |
|
2 |
215 (32.2) |
|
≥3 |
121 (18.1) |
|
No. of cesarean section |
1 |
32 (42.1) |
2 (0–3) |
|
2 |
40 (52.6) |
|
3 |
4 (5.3) |
|
Total |
76 (100) |
|
Pap test |
WNL |
28 (4.2) |
|
|
ASCUS |
181 (27.1) |
|
ASC-H |
45 (6.7) |
|
LSIL |
159 (23.8) |
|
HSIL |
254 (38.1) |
|
Colposcopic-squamocolumnar junction |
Visible |
608 (91.2) |
|
|
Partial visible |
56 (8.4) |
|
Not visible |
3 (0.4) |
|
HPV |
Negative |
54 (8.1) |
|
|
16 |
217 (32.5) |
|
18 |
22 (3.3) |
|
31 |
22 (3.3) |
|
33 |
40 (6.0) |
|
35 |
21 (3.1) |
|
52 |
45 (6.7) |
|
56 |
26 (3.9) |
|
58 |
78 (11.7) |
|
Other high |
69 (10.3) |
|
Unclassified other positive |
26 (3.9) |
|
Low |
47 (7.0) |
|
Diagnosis of punch biopsy |
Normal |
12 (1.8) |
|
|
CIN1 |
73 (10.9) |
|
CIN2 |
235 (35.2) |
|
CIN3 |
297 (44.5) |
|
MIC/SCC |
50 (7.5) |
|
Diagnosis of conization |
Normal |
93 (13.9) |
|
|
CIN1 |
114 (17.1) |
|
CIN2 |
130 (19.5) |
|
CIN3 |
219 (32.8) |
|
MIC/SCC |
111 (16.6) |
|
Final diagnosis |
Normal |
3 (0.4) |
|
|
CIN1 |
58 (8.7) |
|
CIN2 |
191 (28.6) |
|
CIN3 |
281 (42.1) |
|
MIC/SCC |
134 (20.1) |
|
No. of punch biopsy |
1 |
96 (14.4) |
3 (1–9) |
|
2 |
124 (18.6) |
|
3 |
228 (34.2) |
|
4 |
116 (17.4) |
|
5 |
56 (8.4) |
|
≥6 |
47 (7.0) |
|
Interval between punch biopsy and conization (day) |
≤30 |
617 (92.5) |
16 (3–90) |
|
31–60 |
40 (6.0) |
|
61–90 |
10 (1.5) |
|
Discrepancy of diagnosis |
Biopsy overestimation |
224 (59.3) |
|
|
Biopsy underestimation |
154 (40.7) |

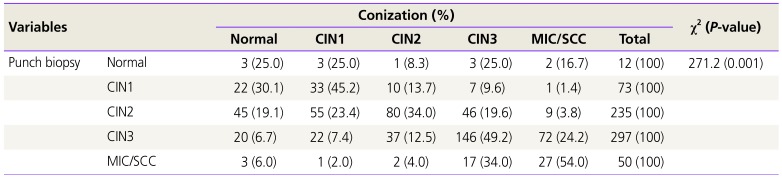
Table 2 shows that the association between the pathologic findings gained by punch biopsy and conization was weak. The overall one-to-one concordance between the punch biopsy and conization histopathology was 43.3%. The rates of detecting a more severe lesion by conization than those by biopsies (biopsy underestimation) were 75.0%, 24.7%, 23.4%, and 24.2% for biopsy results with normal, CIN1, CIN2, and CIN3, respectively. The rates of a less severe lesion detected by conization than those by biopsies (biopsy overestimation) were 30.1%, 42.6%, 26.6%, and 46.0% for biopsy results with CIN1, CIN2, CIN3, and carcinoma, respectively.
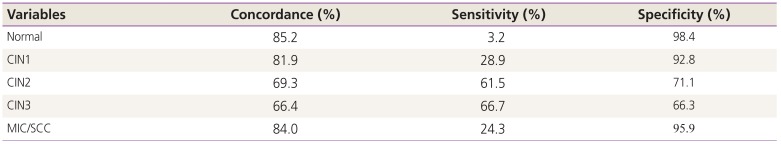
Table 3 shows concordance, sensitivity and specificity for diagnosis of the cervical punch biopsy and conization.
Table 2
Histopathologic comparison of the cervical punch biopsy and conization (n=667)
|
Variables |
Conization (%) |
χ2 (P-value) |
|
Normal |
CIN1 |
CIN2 |
CIN3 |
MIC/SCC |
Total |
|
Punch biopsy |
Normal |
3 (25.0) |
3 (25.0) |
1 (8.3) |
3 (25.0) |
2 (16.7) |
12 (100) |
271.2 (0.001) |
|
CIN1 |
22 (30.1) |
33 (45.2) |
10 (13.7) |
7 (9.6) |
1 (1.4) |
73 (100) |
|
CIN2 |
45 (19.1) |
55 (23.4) |
80 (34.0) |
46 (19.6) |
9 (3.8) |
235 (100) |
|
CIN3 |
20 (6.7) |
22 (7.4) |
37 (12.5) |
146 (49.2) |
72 (24.2) |
297 (100) |
|
MIC/SCC |
3 (6.0) |
1 (2.0) |
2 (4.0) |
17 (34.0) |
27 (54.0) |
50 (100) |

Table 3
Concordance, sensitivity and specificity for diagnosis of the cervical punch biopsy and conization
|
Variables |
Concordance (%) |
Sensitivity (%) |
Specificity (%) |
|
Normal |
85.2 |
3.2 |
98.4 |
|
CIN1 |
81.9 |
28.9 |
92.8 |
|
CIN2 |
69.3 |
61.5 |
71.1 |
|
CIN3 |
66.4 |
66.7 |
66.3 |
|
MIC/SCC |
84.0 |
24.3 |
95.9 |

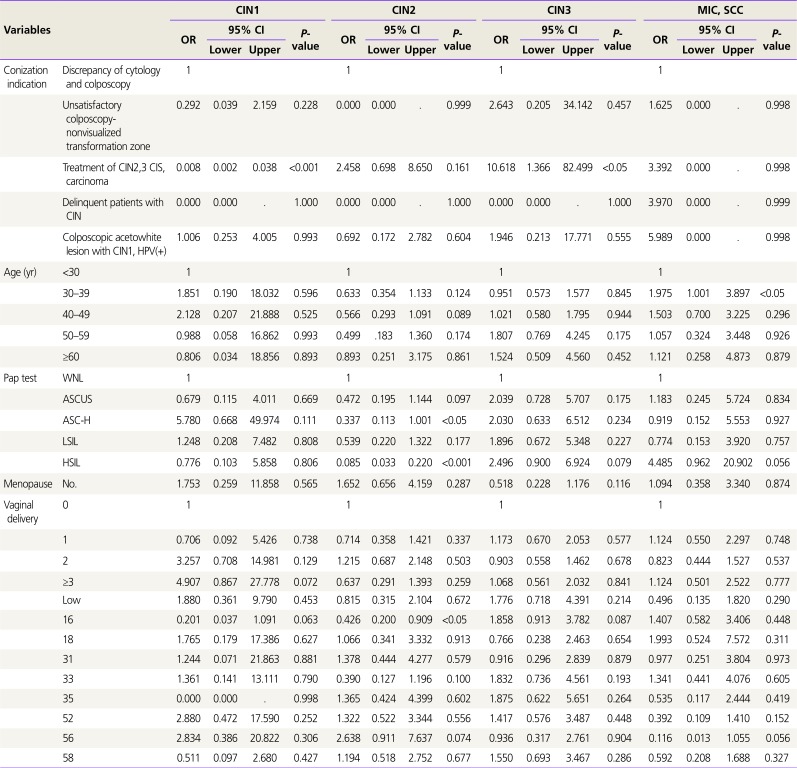
On univariable analysis, indication of conization, age, Pap test, menopausal status, number of vaginal delivery and HPV type were significantly associated with the final diagnosis. Multivariable logistic regression analysis for these variable factors in relation to the final diagnosis shows
Table 4. The factors affecting CIN2 diagnosis are atypical squamous cells cannot exclude HSIL (ASC-H; OR, 0.337,
P<0.05), HSIL (OR, 0.085,
P<0.001), and HPV type 16 (OR, 0.426,
P<0.05), and the factors affecting MIC/squamous cell carcinoma (SCC) diagnosis are reproductive age 30–39 years (OR, 1.975,
P<0.05) and other high HPV types (OR, 0.177,
P<0.05).
Table 4
Multivariable logistic regression analysis for clinical factors affecting final diagnosis
|
Variables |
CIN1 |
CIN2 |
CIN3 |
MIC, SCC |
|
OR |
95% CI |
P-value |
OR |
95% CI |
P-value |
OR |
95% CI |
P-value |
OR |
95% CI |
P-value |
|
Lower |
Upper |
Lower |
Upper |
Lower |
Upper |
Lower |
Upper |
|
Conization indication |
Discrepancy of cytology and colposcopy |
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
|
Unsatisfactory colposcopy-nonvisualized transformation zone |
0.292 |
0.039 |
2.159 |
0.228 |
0.000 |
0.000 |
. |
0.999 |
2.643 |
0.205 |
34.142 |
0.457 |
1.625 |
0.000 |
. |
0.998 |
|
Treatment of CIN2,3 CIS, carcinoma |
0.008 |
0.002 |
0.038 |
<0.001 |
2.458 |
0.698 |
8.650 |
0.161 |
10.618 |
1.366 |
82.499 |
<0.05 |
3.392 |
0.000 |
. |
0.998 |
|
Delinquent patients with CIN |
0.000 |
0.000 |
. |
1.000 |
0.000 |
0.000 |
. |
1.000 |
0.000 |
0.000 |
. |
1.000 |
3.970 |
0.000 |
. |
0.999 |
|
Colposcopic acetowhite lesion with CIN1, HPV(+) |
1.006 |
0.253 |
4.005 |
0.993 |
0.692 |
0.172 |
2.782 |
0.604 |
1.946 |
0.213 |
17.771 |
0.555 |
5.989 |
0.000 |
. |
0.998 |
|
Age (yr) |
<30 |
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
|
30–39 |
1.851 |
0.190 |
18.032 |
0.596 |
0.633 |
0.354 |
1.133 |
0.124 |
0.951 |
0.573 |
1.577 |
0.845 |
1.975 |
1.001 |
3.897 |
<0.05 |
|
40–49 |
2.128 |
0.207 |
21.888 |
0.525 |
0.566 |
0.293 |
1.091 |
0.089 |
1.021 |
0.580 |
1.795 |
0.944 |
1.503 |
0.700 |
3.225 |
0.296 |
|
50–59 |
0.988 |
0.058 |
16.862 |
0.993 |
0.499 |
0.183 |
1.360 |
0.174 |
1.807 |
0.769 |
4.245 |
0.175 |
1.057 |
0.324 |
3.448 |
0.926 |
|
≥60 |
0.806 |
0.034 |
18.856 |
0.893 |
0.893 |
0.251 |
3.175 |
0.861 |
1.524 |
0.509 |
4.560 |
0.452 |
1.121 |
0.258 |
4.873 |
0.879 |
|
Pap test |
WNL |
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
|
ASCUS |
0.679 |
0.115 |
4.011 |
0.669 |
0.472 |
0.195 |
1.144 |
0.097 |
2.039 |
0.728 |
5.707 |
0.175 |
1.183 |
0.245 |
5.724 |
0.834 |
|
ASC-H |
5.780 |
0.668 |
49.974 |
0.111 |
0.337 |
0.113 |
1.001 |
<0.05 |
2.030 |
0.633 |
6.512 |
0.234 |
0.919 |
0.152 |
5.553 |
0.927 |
|
LSIL |
1.248 |
0.208 |
7.482 |
0.808 |
0.539 |
0.220 |
1.322 |
0.177 |
1.896 |
0.672 |
5.348 |
0.227 |
0.774 |
0.153 |
3.920 |
0.757 |
|
HSIL |
0.776 |
0.103 |
5.858 |
0.806 |
0.085 |
0.033 |
0.220 |
<0.001 |
2.496 |
0.900 |
6.924 |
0.079 |
4.485 |
0.962 |
20.902 |
0.056 |
|
Menopause |
No. |
1.753 |
0.259 |
11.858 |
0.565 |
1.652 |
0.656 |
4.159 |
0.287 |
0.518 |
0.228 |
1.176 |
0.116 |
1.094 |
0.358 |
3.340 |
0.874 |
|
Vaginal delivery |
0 |
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
|
1 |
0.706 |
0.092 |
5.426 |
0.738 |
0.714 |
0.358 |
1.421 |
0.337 |
1.173 |
0.670 |
2.053 |
0.577 |
1.124 |
0.550 |
2.297 |
0.748 |
|
2 |
3.257 |
0.708 |
14.981 |
0.129 |
1.215 |
0.687 |
2.148 |
0.503 |
0.903 |
0.558 |
1.462 |
0.678 |
0.823 |
0.444 |
1.527 |
0.537 |
|
≥3 |
4.907 |
0.867 |
27.778 |
0.072 |
0.637 |
0.291 |
1.393 |
0.259 |
1.068 |
0.561 |
2.032 |
0.841 |
1.124 |
0.501 |
2.522 |
0.777 |
|
HPV |
Negative |
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
|
Unclassified other positive |
1.410 |
0.180 |
11.058 |
0.744 |
1.867 |
0.627 |
5.563 |
0.262 |
0.994 |
0.338 |
2.925 |
0.991 |
0.489 |
0.108 |
2.211 |
0.353 |
|
Other high |
1.853 |
0.409 |
8.389 |
0.424 |
1.322 |
0.575 |
3.042 |
0.511 |
1.580 |
0.693 |
3.602 |
0.276 |
0.177 |
0.041 |
0.754 |
<0.05 |
|
Low |
1.880 |
0.361 |
9.790 |
0.453 |
0.815 |
0.315 |
2.104 |
0.672 |
1.776 |
0.718 |
4.391 |
0.214 |
0.496 |
0.135 |
1.820 |
0.290 |
|
16 |
0.201 |
0.037 |
1.091 |
0.063 |
0.426 |
0.200 |
0.909 |
<0.05 |
1.858 |
0.913 |
3.782 |
0.087 |
1.407 |
0.582 |
3.406 |
0.448 |
|
18 |
1.765 |
0.179 |
17.386 |
0.627 |
1.066 |
0.341 |
3.332 |
0.913 |
0.766 |
0.238 |
2.463 |
0.654 |
1.993 |
0.524 |
7.572 |
0.311 |
|
31 |
1.244 |
0.071 |
21.863 |
0.881 |
1.378 |
0.444 |
4.277 |
0.579 |
0.916 |
0.296 |
2.839 |
0.879 |
0.977 |
0.251 |
3.804 |
0.973 |
|
33 |
1.361 |
0.141 |
13.111 |
0.790 |
0.390 |
0.127 |
1.196 |
0.100 |
1.832 |
0.736 |
4.561 |
0.193 |
1.341 |
0.441 |
4.076 |
0.605 |
|
35 |
0.000 |
0.000 |
. |
0.998 |
1.365 |
0.424 |
4.399 |
0.602 |
1.875 |
0.622 |
5.651 |
0.264 |
0.535 |
0.117 |
2.444 |
0.419 |
|
52 |
2.880 |
0.472 |
17.590 |
0.252 |
1.322 |
0.522 |
3.344 |
0.556 |
1.417 |
0.576 |
3.487 |
0.448 |
0.392 |
0.109 |
1.410 |
0.152 |
|
56 |
2.834 |
0.386 |
20.822 |
0.306 |
2.638 |
0.911 |
7.637 |
0.074 |
0.936 |
0.317 |
2.761 |
0.904 |
0.116 |
0.013 |
1.055 |
0.056 |
|
58 |
0.511 |
0.097 |
2.680 |
0.427 |
1.194 |
0.518 |
2.752 |
0.677 |
1.550 |
0.693 |
3.467 |
0.286 |
0.592 |
0.208 |
1.688 |
0.327 |

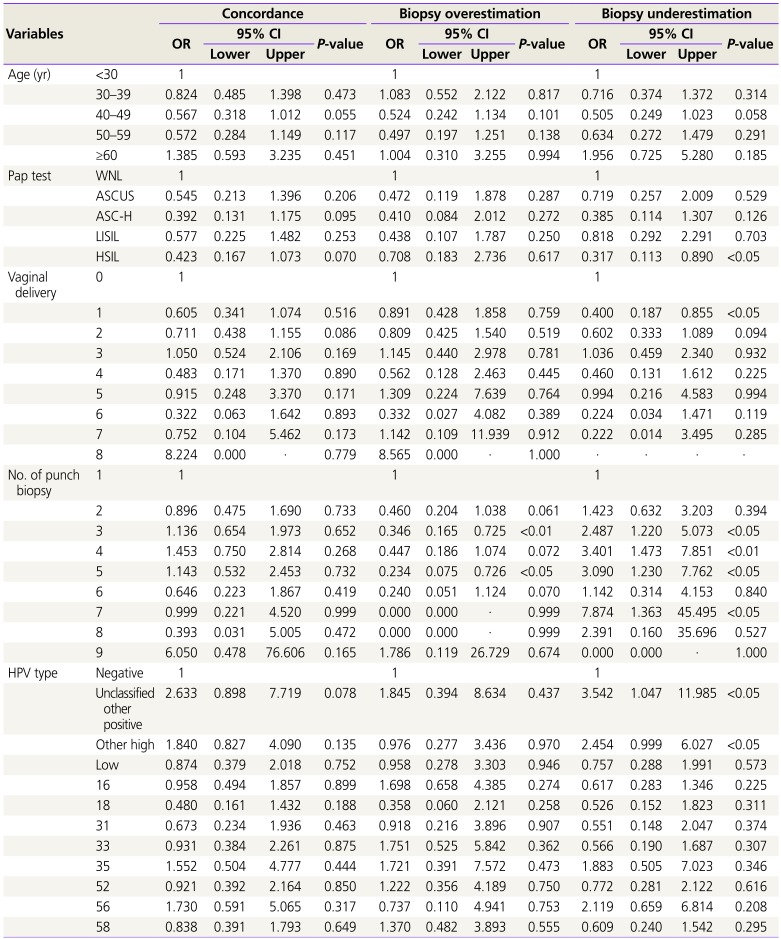
On univariable analysis, involved number of punch biopsy, age, Pap test, number of punch biopsy, number of vaginal delivery, and HPV type were significantly associated with diagnostic discrepancy of punch biopsy and conization.
Table 5 shows multivariable logistic regression analysis for these variable factors affecting discrepancy by dividing them into 3 groups: concordance group, biopsy underestimation, and biopsy overestimation. HSIL at Pap test, <1 vaginal delivery, 3 to 5 biopsies in punch, and unclassified other positive types and other high HPV types were the factors affecting discrepancies, which may result in biopsy underestimation. Three or 5 punch biopsy number is also a significant factor of biopsy overestimation.
Table 5
Multivariable logistic regression analysis for clinical factors affecting diagnostic discrepancy of punch biopsy and conization
|
Variables |
Concordance |
Biopsy overestimation |
Biopsy underestimation |
|
OR |
95% CI |
P-value |
OR |
95% CI |
P-value |
OR |
95% CI |
P-value |
|
Lower |
Upper |
Lower |
Upper |
Lower |
Upper |
|
Age (yr) |
<30 |
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
|
30–39 |
0.824 |
0.485 |
1.398 |
0.473 |
1.083 |
0.552 |
2.122 |
0.817 |
0.716 |
0.374 |
1.372 |
0.314 |
|
40–49 |
0.567 |
0.318 |
1.012 |
0.055 |
0.524 |
0.242 |
1.134 |
0.101 |
0.505 |
0.249 |
1.023 |
0.058 |
|
50–59 |
0.572 |
0.284 |
1.149 |
0.117 |
0.497 |
0.197 |
1.251 |
0.138 |
0.634 |
0.272 |
1.479 |
0.291 |
|
≥60 |
1.385 |
0.593 |
3.235 |
0.451 |
1.004 |
0.310 |
3.255 |
0.994 |
1.956 |
0.725 |
5.280 |
0.185 |
|
Pap test |
WNL |
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
|
ASCUS |
0.545 |
0.213 |
1.396 |
0.206 |
0.472 |
0.119 |
1.878 |
0.287 |
0.719 |
0.257 |
2.009 |
0.529 |
|
ASC-H |
0.392 |
0.131 |
1.175 |
0.095 |
0.410 |
0.084 |
2.012 |
0.272 |
0.385 |
0.114 |
1.307 |
0.126 |
|
LISIL |
0.577 |
0.225 |
1.482 |
0.253 |
0.438 |
0.107 |
1.787 |
0.250 |
0.818 |
0.292 |
2.291 |
0.703 |
|
HSIL |
0.423 |
0.167 |
1.073 |
0.070 |
0.708 |
0.183 |
2.736 |
0.617 |
0.317 |
0.113 |
0.890 |
<0.05 |
|
Vaginal delivery |
0 |
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
|
1 |
0.605 |
0.341 |
1.074 |
0.516 |
0.891 |
0.428 |
1.858 |
0.759 |
0.400 |
0.187 |
0.855 |
<0.05 |
|
2 |
0.711 |
0.438 |
1.155 |
0.086 |
0.809 |
0.425 |
1.540 |
0.519 |
0.602 |
0.333 |
1.089 |
0.094 |
|
3 |
1.050 |
0.524 |
2.106 |
0.169 |
1.145 |
0.440 |
2.978 |
0.781 |
1.036 |
0.459 |
2.340 |
0.932 |
|
4 |
0.483 |
0.171 |
1.370 |
0.890 |
0.562 |
0.128 |
2.463 |
0.445 |
0.460 |
0.131 |
1.612 |
0.225 |
|
5 |
0.915 |
0.248 |
3.370 |
0.171 |
1.309 |
0.224 |
7.639 |
0.764 |
0.994 |
0.216 |
4.583 |
0.994 |
|
6 |
0.322 |
0.063 |
1.642 |
0.893 |
0.332 |
0.027 |
4.082 |
0.389 |
0.224 |
0.034 |
1.471 |
0.119 |
|
7 |
0.752 |
0.104 |
5.462 |
0.173 |
1.142 |
0.109 |
11.939 |
0.912 |
0.222 |
0.014 |
3.495 |
0.285 |
|
8 |
8.224 |
0.000 |
. |
0.779 |
8.565 |
0.000 |
. |
1.000 |
. |
. |
. |
. |
|
No. of punch biopsy |
1 |
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
|
2 |
0.896 |
0.475 |
1.690 |
0.733 |
0.460 |
0.204 |
1.038 |
0.061 |
1.423 |
0.632 |
3.203 |
0.394 |
|
3 |
1.136 |
0.654 |
1.973 |
0.652 |
0.346 |
0.165 |
0.725 |
<0.01 |
2.487 |
1.220 |
5.073 |
<0.05 |
|
4 |
1.453 |
0.750 |
2.814 |
0.268 |
0.447 |
0.186 |
1.074 |
0.072 |
3.401 |
1.473 |
7.851 |
<0.01 |
|
5 |
1.143 |
0.532 |
2.453 |
0.732 |
0.234 |
0.075 |
0.726 |
<0.05 |
3.090 |
1.230 |
7.762 |
<0.05 |
|
6 |
0.646 |
0.223 |
1.867 |
0.419 |
0.240 |
0.051 |
1.124 |
0.070 |
1.142 |
0.314 |
4.153 |
0.840 |
|
7 |
0.999 |
0.221 |
4.520 |
0.999 |
0.000 |
0.000 |
. |
0.999 |
7.874 |
1.363 |
45.495 |
<0.05 |
|
8 |
0.393 |
0.031 |
5.005 |
0.472 |
0.000 |
0.000 |
. |
0.999 |
2.391 |
0.160 |
35.696 |
0.527 |
|
9 |
6.050 |
0.478 |
76.606 |
0.165 |
1.786 |
0.119 |
26.729 |
0.674 |
0.000 |
0.000 |
. |
1.000 |
|
HPV type |
Negative |
1 |
|
|
|
1 |
|
|
|
1 |
|
|
|
|
Unclassified other positive |
2.633 |
0.898 |
7.719 |
0.078 |
1.845 |
0.394 |
8.634 |
0.437 |
3.542 |
1.047 |
11.985 |
<0.05 |
|
Other high |
1.840 |
0.827 |
4.090 |
0.135 |
0.976 |
0.277 |
3.436 |
0.970 |
2.454 |
0.999 |
6.027 |
<0.05 |
|
Low |
0.874 |
0.379 |
2.018 |
0.752 |
0.958 |
0.278 |
3.303 |
0.946 |
0.757 |
0.288 |
1.991 |
0.573 |
|
16 |
0.958 |
0.494 |
1.857 |
0.899 |
1.698 |
0.658 |
4.385 |
0.274 |
0.617 |
0.283 |
1.346 |
0.225 |
|
18 |
0.480 |
0.161 |
1.432 |
0.188 |
0.358 |
0.060 |
2.121 |
0.258 |
0.526 |
0.152 |
1.823 |
0.311 |
|
31 |
0.673 |
0.234 |
1.936 |
0.463 |
0.918 |
0.216 |
3.896 |
0.907 |
0.551 |
0.148 |
2.047 |
0.374 |
|
33 |
0.931 |
0.384 |
2.261 |
0.875 |
1.751 |
0.525 |
5.842 |
0.362 |
0.566 |
0.190 |
1.687 |
0.307 |
|
35 |
1.552 |
0.504 |
4.777 |
0.444 |
1.721 |
0.391 |
7.572 |
0.473 |
1.883 |
0.505 |
7.023 |
0.346 |
|
52 |
0.921 |
0.392 |
2.164 |
0.850 |
1.222 |
0.356 |
4.189 |
0.750 |
0.772 |
0.281 |
2.122 |
0.616 |
|
56 |
1.730 |
0.591 |
5.065 |
0.317 |
0.737 |
0.110 |
4.941 |
0.753 |
2.119 |
0.659 |
6.814 |
0.208 |
|
58 |
0.838 |
0.391 |
1.793 |
0.649 |
1.370 |
0.482 |
3.893 |
0.555 |
0.609 |
0.240 |
1.542 |
0.295 |

Go to :

Discussion
The colposcopic characteristics have been used to identify areas with the highest degree of visual abnormality, and colposcopically directed biopsy obtained from those areas have been the criterion standard of diagnostic procedure for the diagnosis of cervical precancerous lesions. However, the development of LEEP provided the opportunity to assess the accuracy of colposcopy. A review of literature noted a 42% to 57% concordance rate of diagnostic pathology between colposcopically directed biopsy and conization, and overall sensitivity was 50–70% and 55–90% for CIN1 and CIN2/3, respectively, and overall specificity was 80% and 96% for CIN1 and CIN2/3, respectively [
1]. The biopsy underestimation rates were 71.42% for negative, 22.91% for CIN1, 37.03% for CIN2, and 12.72% for CIN3/carcinoma
in situ, and biopsy overestimation was 29.16% for CIN1, 40.74% for CIN2, 15.45% for CIN3/carcinoma
in situ lesions [
11]. Our study showed similar results as 43.3% of overall agreement in punch biopsy and LEEP pathology. The overall rates of biopsy underestimation were 23.1%, and 75.0% in normal, 24.7% in CIN1, 23.4%, in CIN2 and 24.2% in CIN3. The overall rates of biopsy overestimation were 33.6%, and 30.1% in CIN1, 42.6% in CIN2, 26.6% in CIN3 and 46.0% in MIC/SCC. Stoler et al. [
8] published an interesting study, with an overall concordance rate, biopsy underestimation, and biopsy overestimation of 42%, 21%, and 36%, respectively. Age, number of biopsies, lesion size, and infection of HPV 16/18 influenced overall agreement, and the number of biopsies and HPV 16/18 status affect biopsy underestimation. In a previous report, observations regarding positive association of patient age, invisibility of squamocolumnar junction, number of quadrants involved, and cone width with the probability of non-diagnosis of carcinoma were confirmed [
12].
In our study, the analysis of the final diagnosis represented that age 30 to 39 years (OR, 1.975,
P<0.05) and other high HPV types (OR, 0.177,
P<0.05) were associated with cancer diagnosis, whereas ASC-H (OR, 0.337,
P<0.05), HSIL (OR, 0.085,
P<0.001), and HPV type 16 (OR, 0.426,
P<0.05) affected the diagnosis of CIN2 (
Table 4).
In previous reports, we can observe a positive association between the patient's age and risk of abnormal lesions. It is increased 4.5 times for those older than 30 years, and 11 times for those older than 50 years [
12]. Chen et al. [
13] observed an increase in risks for patients older than 45 years. One report compares women younger than and older than 35 years old and found that the patients in the younger cohort were 2.6 times as likely to have any squamous intraepithelial lesion on evaluation and 2.9 times as likely to have high-grade changes [
14]. This would indicate that patients over the age of 35 years may have some decreased risk, but this has not been widely reported. Some authors reporting a reduced or similar overall risk of dysplasia as that reported in younger women [
1516] and others reporting an increased risk [
17].
As hypothesised by Wetrich [
18], the most plausible explanation is the underlying age trend in the incidence of invasive carcinoma [
12]. Because of its association with chronic cervicitis, patient age is also likely to influence the probability of micro invasion being mistaken for squamous metaplasia of the cervical glands and reactive atypia. In menopause, poor cell maturation may further complicate the differential diagnosis. Evaluation of the postmenopausal cervix is difficult, given the tendency for the transition zone to recede into the cervical canal with removal of estrogen stimulation. This makes adequate cytologic sampling a challenge and satisfactory colposcopic evaluation often difficult. Our result emphasizes that co-testing using the combination of Pap cytology plus HPV DNA testing is the preferred cervical cancer screening method. However, it is still difficult to generalize our results with age and more research is needed.
In our study, the final diagnosis of ASC-H or HSIL population showed more CIN3 or SCC than CIN2. Partial or total invisibility of the squamocolumnar junction [
19] and error by the colposcopist in selecting a biopsy site in a large surface lesion [
20] have often been postulated to explain nondiagnosis of carcinoma by biopsy. Also, colposcopic biopsy fail to detect 30% to 50% of prevalent HSILs [
2122]. In women with HPV types 16 and 18 in whom a normal Pap smear was obtained, the probability of developing precancerous cervical lesions is 35 times higher [
23]. Women with negative pre-conization high risk-HPV test results or a low viral load have a high probability of having no lesion in the conization specimen [
4]. In patient with ASC-H, HSIL, and HPV type 16, colposcopy directed biopsy may be neither a good diagnostic nor a reliable management method and immediately conization without punch biopsy can avoid overlooking high-grade lesions.
The biopsy underestimation of normal/CIN1 is 31.8%, that is, the LEEP pathology is CIN2, CIN3, or MIC/SCC. These findings raise questions regarding the accuracy of colposcopy and lesion progression to high-grade disease. Our data has demonstrated that <1 vaginal delivery (OR, 0.40,
P<0.05), HSIL (OR, 0.317,
P<0.05), number of punch biopsies (OR, 2.487,
P<0.05 at 3 biopsies; OR, 3.401,
P<0.01 at 4 biopsies; OR, 3.090,
P<0.05 at 5 biopsies), and HPV type (OR, 2.454,
P<0.05 at other high types; OR, 3.542,
P<0.05 at unclassified other positive types) were significant factors of biopsy underestimation (
Table 5). Unusually, cesarean section was shown to have no effects, but vaginal delivery is one of the discrepancy factors, which can be assumed to affect squamocolumnar junction unlike cesarean section. A previous report showed nulliparity as a significant risk factor for CIN in patients with HIV (1.42; 95% CI, 1.01–1.99) [
24]. Several papers showed that the higher number of colposcopically directed biopsies improves the detection rates (47% for one biopsy, 65% for 2 biopsies, and 77% for 3 or more biopsies,
P=0.004) [
825]. These data also suggest that taking more biopsies increases the detection of HSILs. Some investigators have proposed that taking biopsies of normal-appearing cervix may identify up to 30% more HSILs [
2627]. In recent biopsy study, adding a second
Biopsy increased the sensitivity to detect a prevalent HSIL significantly from 61% to 86%. Adding a third biopsy increased the sensitivity further to 96%. The incremental benefit of taking multiple biopsies was present regardless of referral cytology, HPV-16 status, and colposcopic impression [
28]. Our result for number of punch biopsy support previous data and we recommend multiple biopsy.
Other high HPV types, including types 39, 45, 51, 53, 59, 66, 68, and 73 show a low frequency rate and can cause biopsy underestimation. Therefore, more attention should be paid to the punch biopsy when other high HPV types are positive. HPV test is less subjective and more sensitive in detecting precancerous lesions than cytology alone. HPV test was found to demonstrate a mean of 35.7% greater sensitivity than cytology alone in the detection of CIN2/3 [
29]. HSIL requires pathologic confirmation to be diagnosed. This suggests that punch biopsy and LEEP conization may be helpful by performing Pap test and HPV test together. Other studies showed that the transformation zone is difficult to detect in postmenopausal women, a sit recedes into the cervical canal and may not obtain the precise cytology and/or pathology. Moore et al. [
30] showed that 30% of all patients older than 50 years had unsatisfactory colposcopy, and 50% of those patients showed discrepancy between cytology and colposcopic pathology. They recommend LEEP conization in these cases of discrepancy between cytology and colposcopic biopsy.
The biopsy overestimation can occur due to the fact that the remaining small lesions after punch biopsy may undergo spontaneous regression. Spontaneous regression of CIN2/3 after punch biopsy has been estimated to occur in up to 20% of patients [
31].
Previous reports present several hypotheses regarding histopathologic discrepancy [
363233]. First, it may be that the high-grade CIN was only a small lesion that was completely removed by the biopsy and/or ECC. Second, CINs may be missed and not removed by LEEP. In this group, the lesions either probably remained in the endocervix or multifocal and outside the resection margin. Third, the cervical biopsy and/or cone specimen were misdiagnosed. The pathologist may fail to correctly diagnose contained CIN or carcinoma because the lesion may include from normal to carcinoma. In our study, the number of punch biopsies (OR, 0.346,
P<0.01 at 3 biopsies; OR, 0.234,
P<0.05 at 5 biopsies) is a unique factor of biopsy overestimation. Three or 5 biopsies may help in deciding using LEEP conization, considering lesion size by colposcopic finding.
Many patients, whose colposcopically directed biopsy and LEEP conization showed pathologic discrepancies, may be overtreated at the circumstance of biopsy overestimation or experience undue stress as they might develop cervical cancer in cases of biopsy underestimation. Therefore, investigation of the factors causing the discrepancy in the 2 steps is important. In the present study, we found that the number of vaginal delivery, HPV type, number of punch biopsies, and severity of cervical cytology seems to be a clinical factor for discrepancy. Our study shows that Pap test (ASC-H, HSIL) and HPV type 16 for the final diagnosis of CIN2, and HPV other high types and positive for the final diagnosis of cancer are significant factors of affecting the discrepancy in the 2 steps. This suggests that punch biopsy and LEEP conization may be helpful in performing the test by performing Pap test and HPV test together. Ultimately, we proposed to implement discrepancy of punch biopsy and LEEP conization, considering the number of vaginal delivery, HPV type (particularly other high types and positive), severity of cervical cytology, number of punch biopsies, particularly 3 more and age (
Table 5).
The present study has several limitations. They are retrospective in design, small number of cases, and the fact that all procedures were performed by 5 physicians, which might have introduced bias into the study. Another limitation was the omission of data for the endocervical curettage (ECC), the lack of some clinical variables such as tobacco use or colposcopic experience of physicians, and the lack of the size of cervical lesions and LEEP specimens. Based on the ASCCP, ECC should be performed in specific situations, such as unsatisfactory colposcopy following low-grade lesion, colposcopic evaluation of high-grade lesion, or initial evaluation of all subcategories of atypical glandular cell cytology. There is an argument that ECC can be of limited benefit if excisional treatment is recommended. In addition, the value of the procedure remains controversial [
34]. We also suggest that an accurate Pap test can reduce the discrepancy of 2 steps. An accurate punch biopsy and follow-up at a relatively low frequency of HPV high type is also recommended. We will proceed with further studies on how to obtain accurate cytological findings and examine the risks of other high HPV types.
In conclusion, ASC-H, HSIL, and HPV type 16 affect CIN2 diagnosis, and the factors affecting cancer diagnosis are age 30 to 39 years and other high HPV types. Simultaneous Pap test and HPV test can reduce unnecessary conizations, and reproductive age and HPV infection of other high types should be considered for determination whether follow-up or conization should be performed. The number of punch biopsy is a unique factor of biopsy under- and overestimation. We recommend 3 or 5 colposcopic punch biopsies to reduce unnecessary conization and increase its value for cancer diagnosis.
Go to :










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download