Abstract
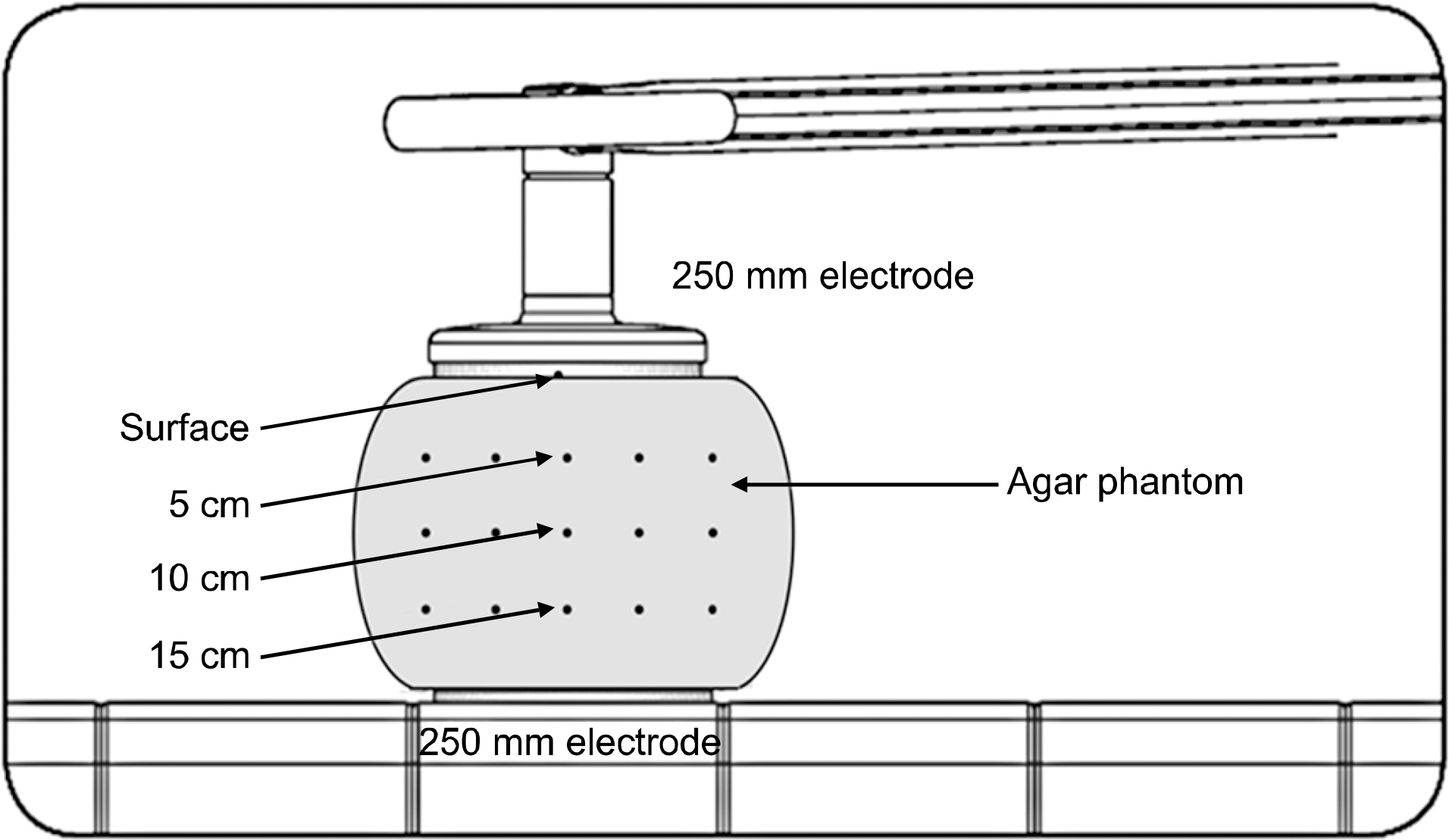
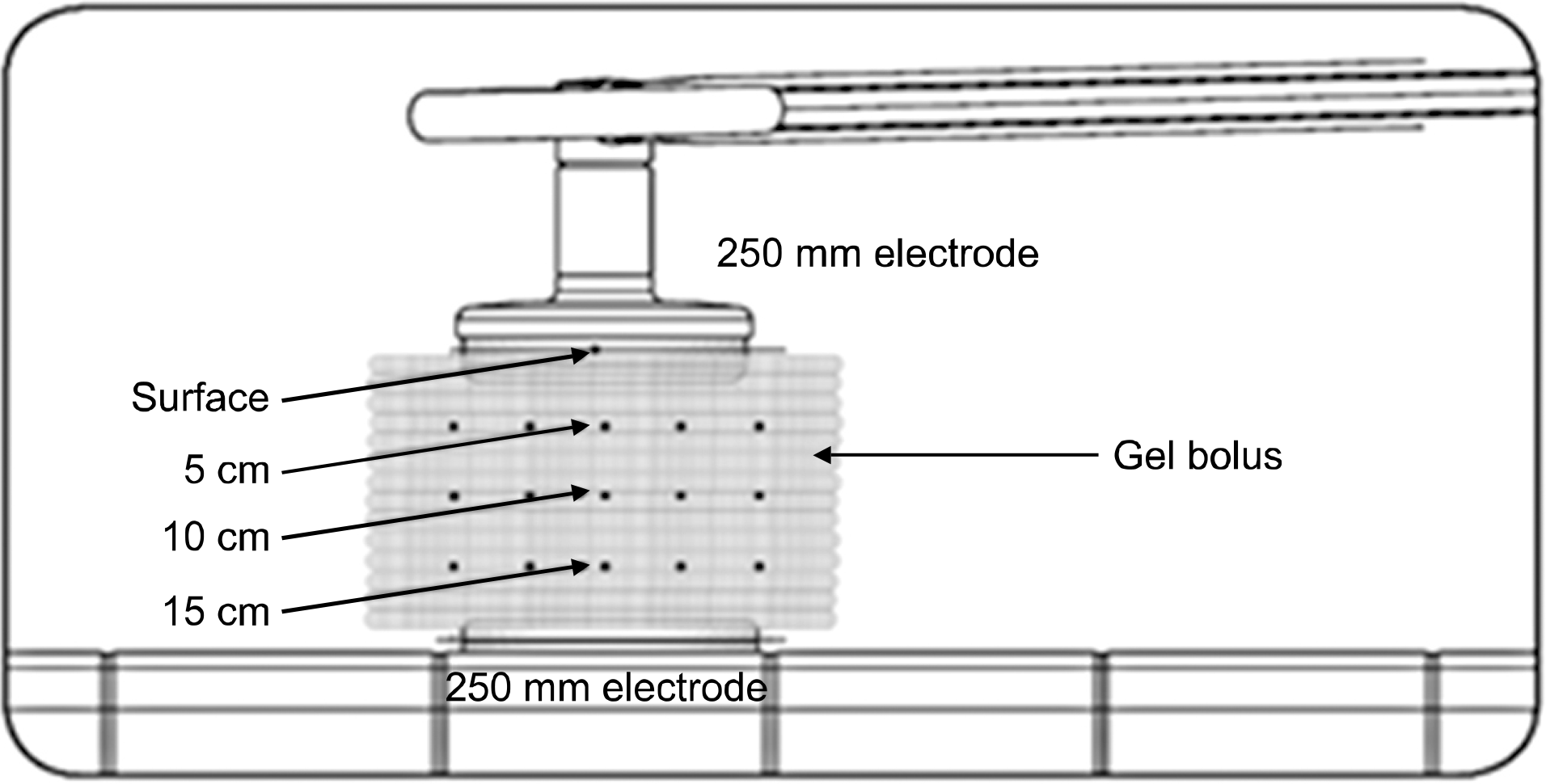
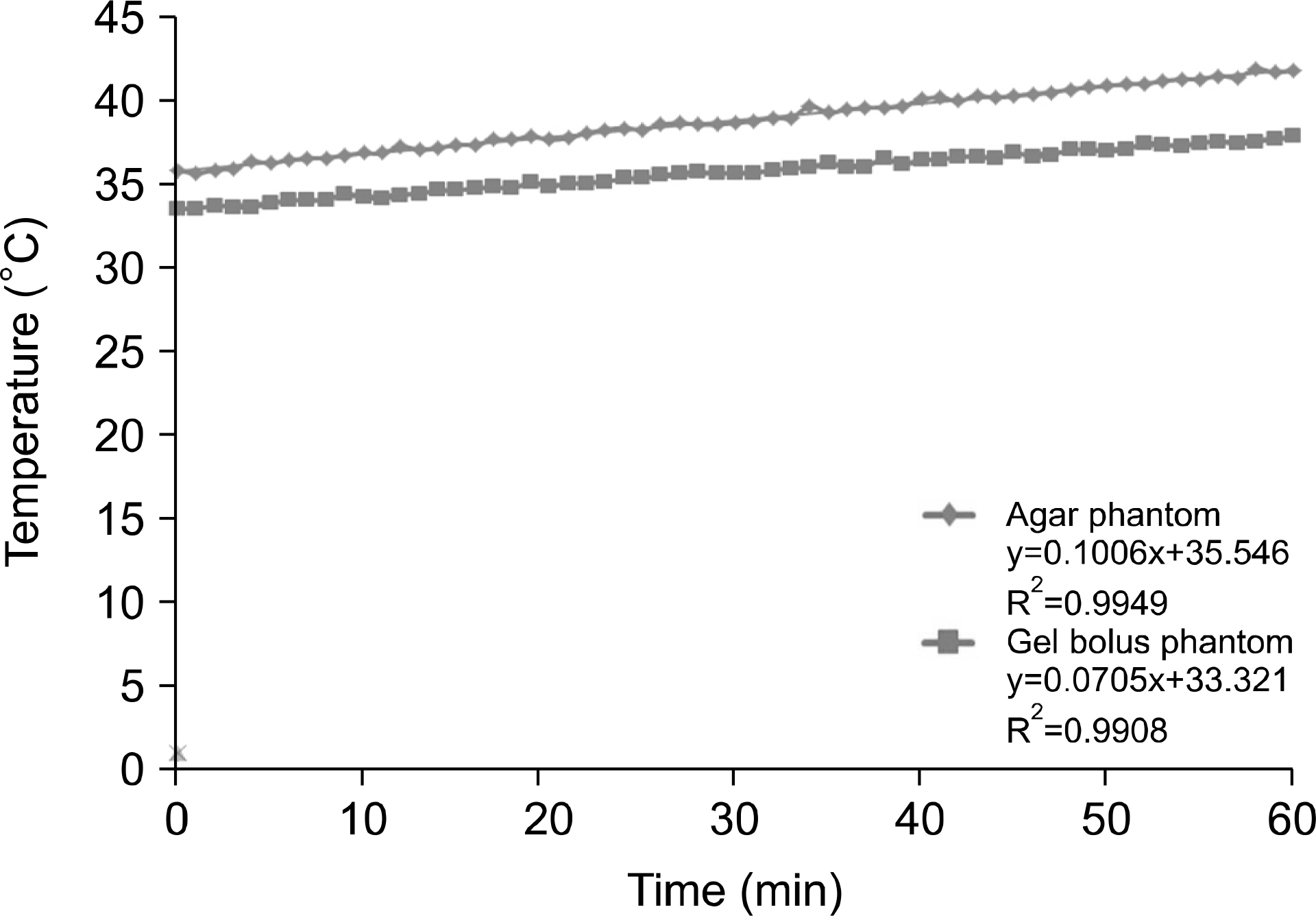
The usefulness of Gel Bolus phantom was investigated by comparing the temperature distribution characteristic of the agar phantom produced to investigate the dose distribution characteristic of radiofrequency hyperthermia device with that of the Gel Bolus phantom under conditions similar to those of an agar phantom that can continuously carry out temperature measurement. The temperatures of the agar phantom and the Gel Bolus phantom were raised to 36.5±3oC and a temperature sensing was inserted at depths of 5, 10, and 15 cm from the phantom central axis. The temperature increase rate and the coefficient of determination were analyzed while applying output powers of 100 W and 150 W, respectively, at intervals of 1 min for 60 min under conditions where the indoor temperature was in the range 24.5∼27.5oC, humidity was 35∼40%, internal cooling temperature of the electrode was 20oC, size of the upper electrode was 250 mm, and the size of the lower electrode was 250 mm. The coefficients of determination of 150 W output power at the depth point of 5 cm from the central axis of the phantom were analyzed to be 0.9946 and 0.9926 in the agar and Gel Bolus phantoms, respectively; moreover, the temperature change equation of the agar and Gel Bolus phantoms with time can be expressed as follows in the state the phantom temperature is raised to 36oC: Y(G) is equation of Gel Bolus phantoms (in 5 cm depth) applying output power of 150 W. Y(G)=0.157X+36. It can be seen that if the temperature is measured in this case, the Gel Bolus phantom value can be converted to the measured value of the agar phantom. As a result of comparing the temperature distribution characteristics of the agar phantom of a human-body-equivalent material with those of the Gel Bolus phantom that can be continuously used, the usefulness of Gel Bolus phantom was exhibited.
Go to : 
REFERENCES
1. Byun YJ, Lee EH, Lee JP, Chang KH, Ryu HS. The effect of hormone replacement therapy on osteoporosis following irradiation of gynecologic cancer patients. J Gynecol Oncol. 16(1):53–60. 2005.
2. Chung IY, Kim EJ, Park JM, Yoo JM. Results of combined chemotherapy and radiotherapy for advanced intraocular retinoblastoma, Korean J Ophthalmol. 44(7):1528–1537. 2003.
3. Kim MO. Concurrent chemoradiotherapy of locally advanced pancreatic carcinoma and effects of hyperthermia. Korean Cancer Association. 26(4):561–566. 1994.
4. Cui ZG, Piao JL, Rehman MU, Ogawa R, Li P, Zhao QL, et al. Molecular mechanisms of hyperthermia-induced apoptosis enhanced by withaferin A. Eur J Pharmacol. 15(723):99–107. 2014.

5. Hatashita M, Taniguchi M, Baba K, Koshiba K, Sato T, Jujo Y, et al. Sinodielide A exerts thermosensitizing effects and induces apoptosis and G2/M cell cycle arrest in DU145 human prostate cancer cells via the Ras/Raf/MAPK and PI3K/Akt signaling pathways. Int J Mol Med. 33(2):406–414. 2014.

6. Song X, Kim SY, Lee YJ. Evidence for two modes of synergistic induction of apoptosis by mapatumumab and oxaliplatin in combination with hyperthermia in human colon cancer cells. PLoS One. 8(8):e73654. 2013.

7. Jiang W, Bian L, Wang N, He Y. Proteomic analysis of protein expression profiles during hyperthermia-induced apoptosis in Tca8113 cells. Oncol Lett. 6(1):135–143. 2013.

8. Jang HS. Clinical trial of concomitant thermo-chemotherapy in cervix cancer patients. Korean Cancer Association. 27(6):968–977. 1995.
9. Suh CO, Loh JK, Seong JS, Roh JK, Kim BS, Park IS, et al. Effect of radiofrequency hyperthermia on hepatocellular carcinoma. Korean Cancer Association. 20(2):117–125. 1988.
10. Solopan S, Belous A, Yelenich A, Bubnovskaya L, Kovelskaya A, Podoltsev A, et al. Lanthanum-strontium manganite magnetic fluid as potential inducer of tumor hyperthermia. Exp Oncol. 33(3):130–135. 2011.
11. Lui PC, Fan YS, Xu G, Ngai CY, Fung KP, Tse GM, et al. Apoptotic and necrotic effects of tumour necrosis factor-alpha potentiated with hyperthermia on L929 and tumour necrosis factor-alpha-resistant L929. Int J Hyperthermia. 26(6):556–564. 2010.

12. Thews O, Lambert C, Kelleher DK, Biesalski HK, Vaupel P, Frank J. Impact of therapeutically induced reactive oxygen species and radical scavenging by alpha-tocopherol on tumor cell adhesion. Oncol Rep. 18(4):965–971. 2007.
13. Ahmed K, Zhao QL, Matsuya Y, Yu DY, Salunga TL, Nemoto H, et al. Enhancement of macrosphelide-induced apoptosis by mild hyperthermia. Int J Hyperthermia. 23(4):353–361. 2007.

14. Sikora M, Gese A, Czypicki R, Gsior M, Tretyn A, Chojnowski J, et al. Correlations between polymorphisms in genes coding elements of dopaminergic pathways and body mass index in overweight and obese women. Endokrynol Pol. 64(2):101–107. 2013.
Go to : 
 | Fig. 1.Making of agar phantom: (a) Agar phantom mold, (b) Agar powder of MSC Co. Ltd, (c) Sterile water, (d) Gel agar solidified with phantom, (e) Gel agar phantom separated from the mold, (f) Completed production of agar phantom. |
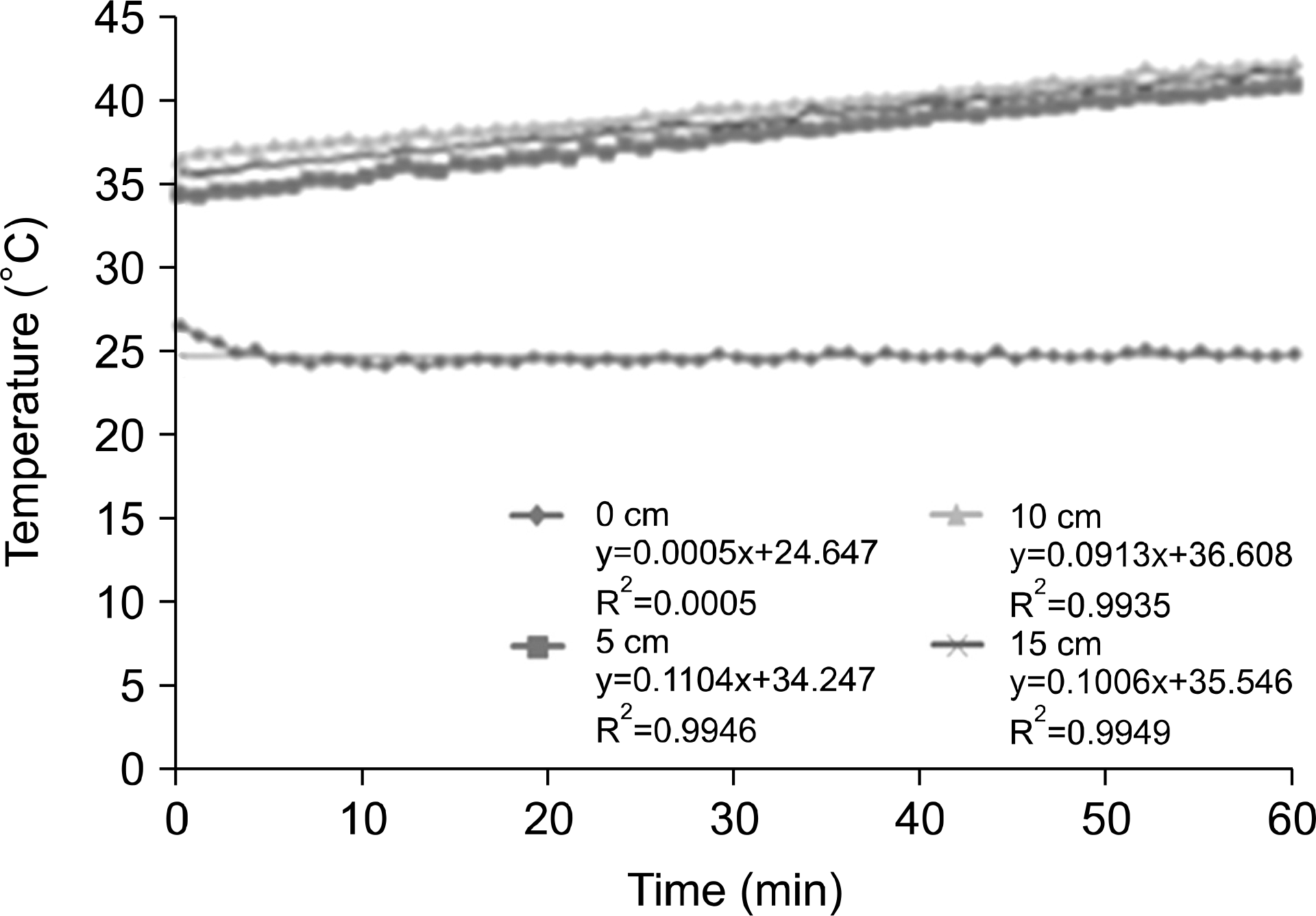 | Fig. 5.Depth of measurement temperature on the agar phantom for 60 min at 100 W output power. |
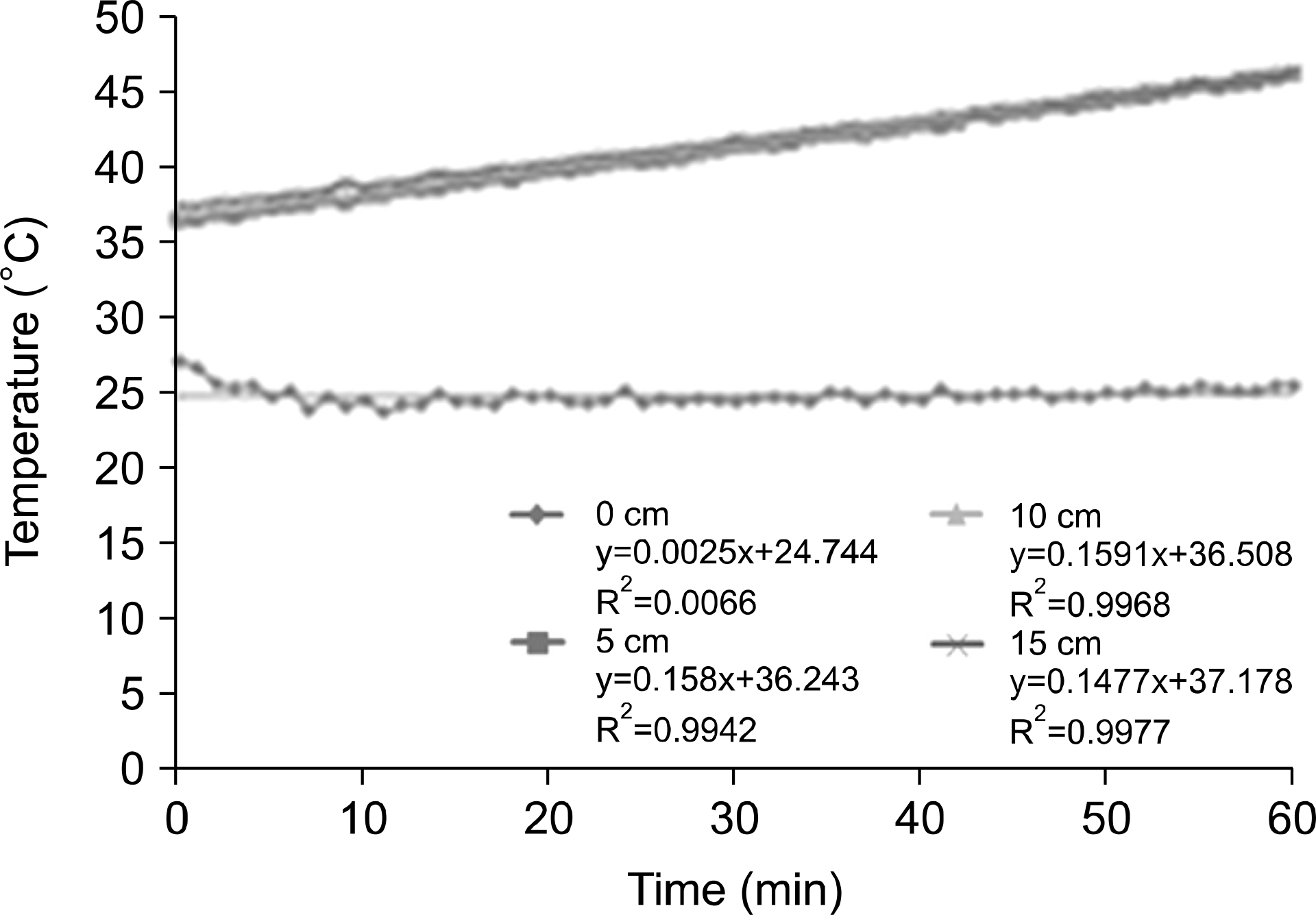 | Fig. 6.Depth of measurement temperature on the agar phantom for 60 min at 150 W output power. |
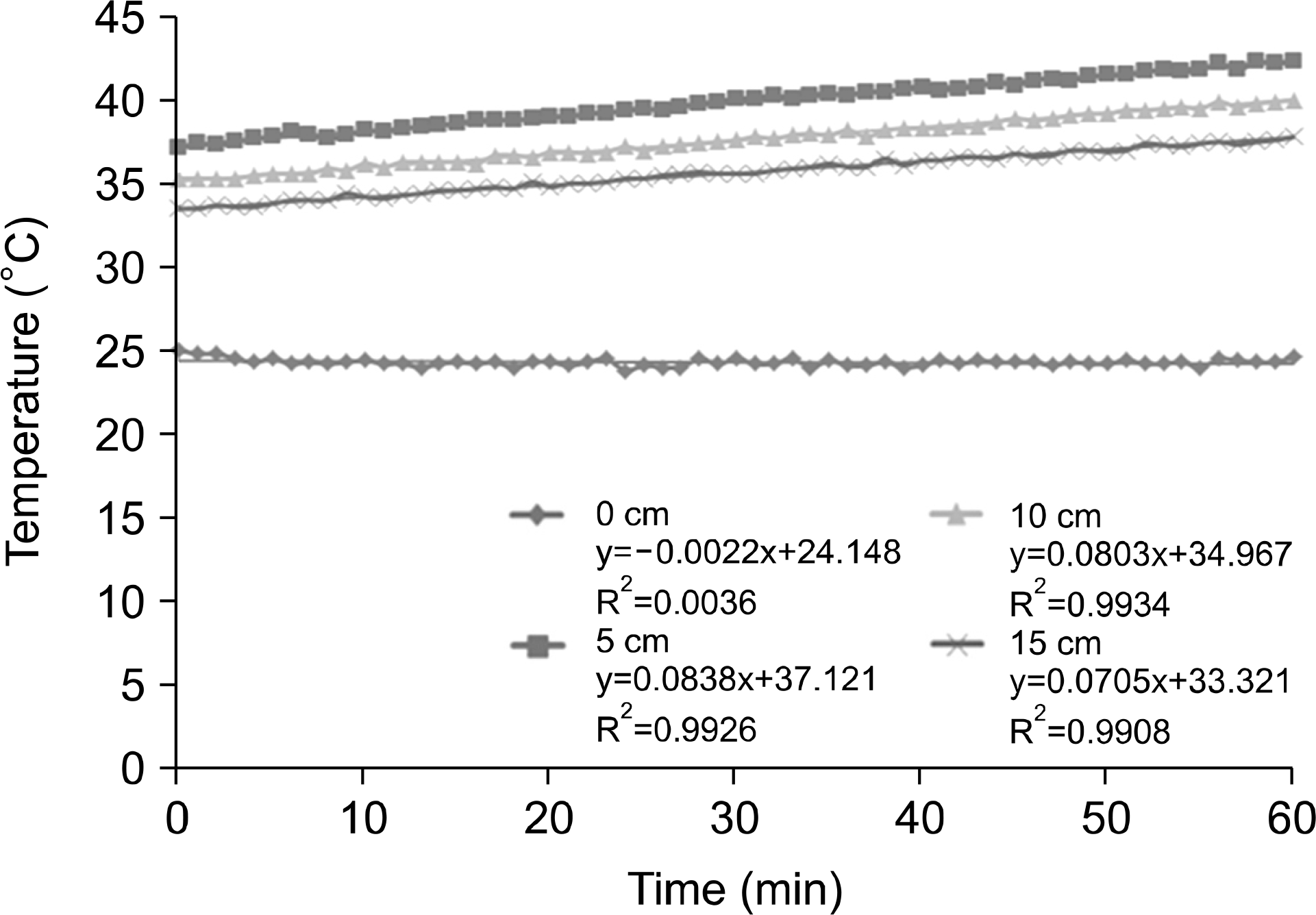 | Fig. 7.Depth of measurement temperature on the gel bolus phantom for 60 min at 100 W output power. |
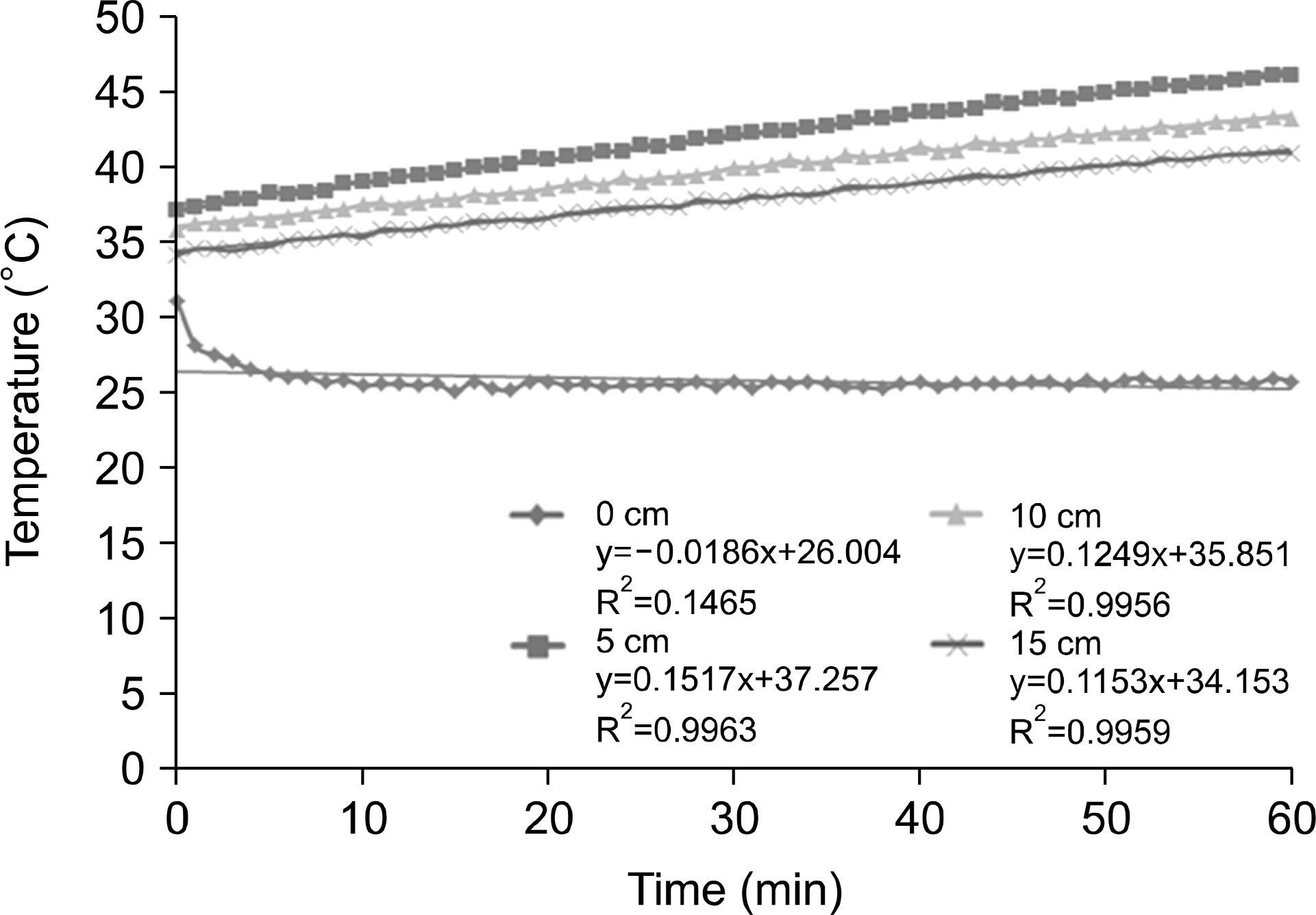 | Fig. 8.Depth of measurement temperature on gel bolus phantom for 60 min at 150 W output power. |
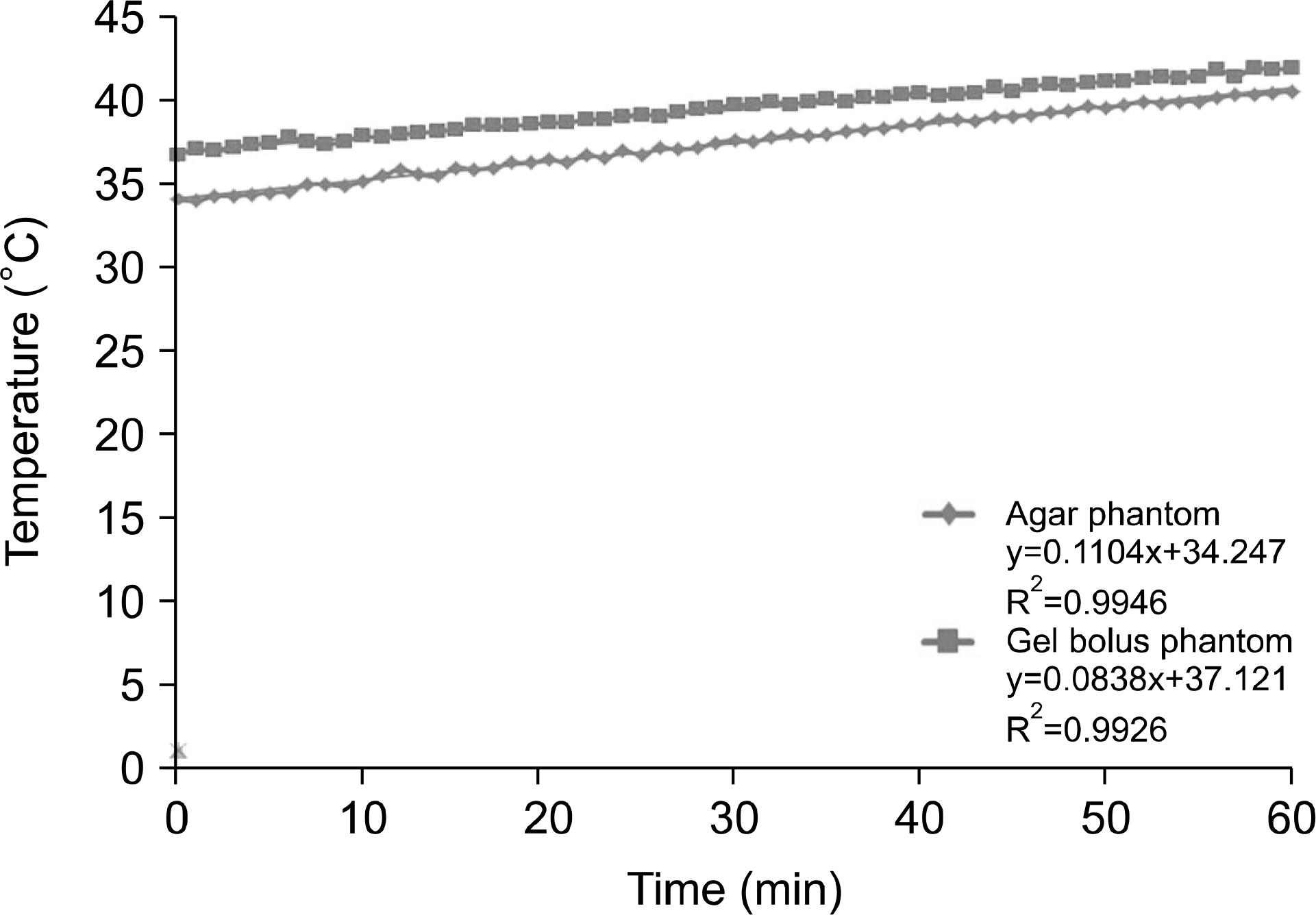 | Fig. 9.5-cm depth for 60 min at 100 W output power for the temperature analysis of the agar and gel bolus phantoms. |
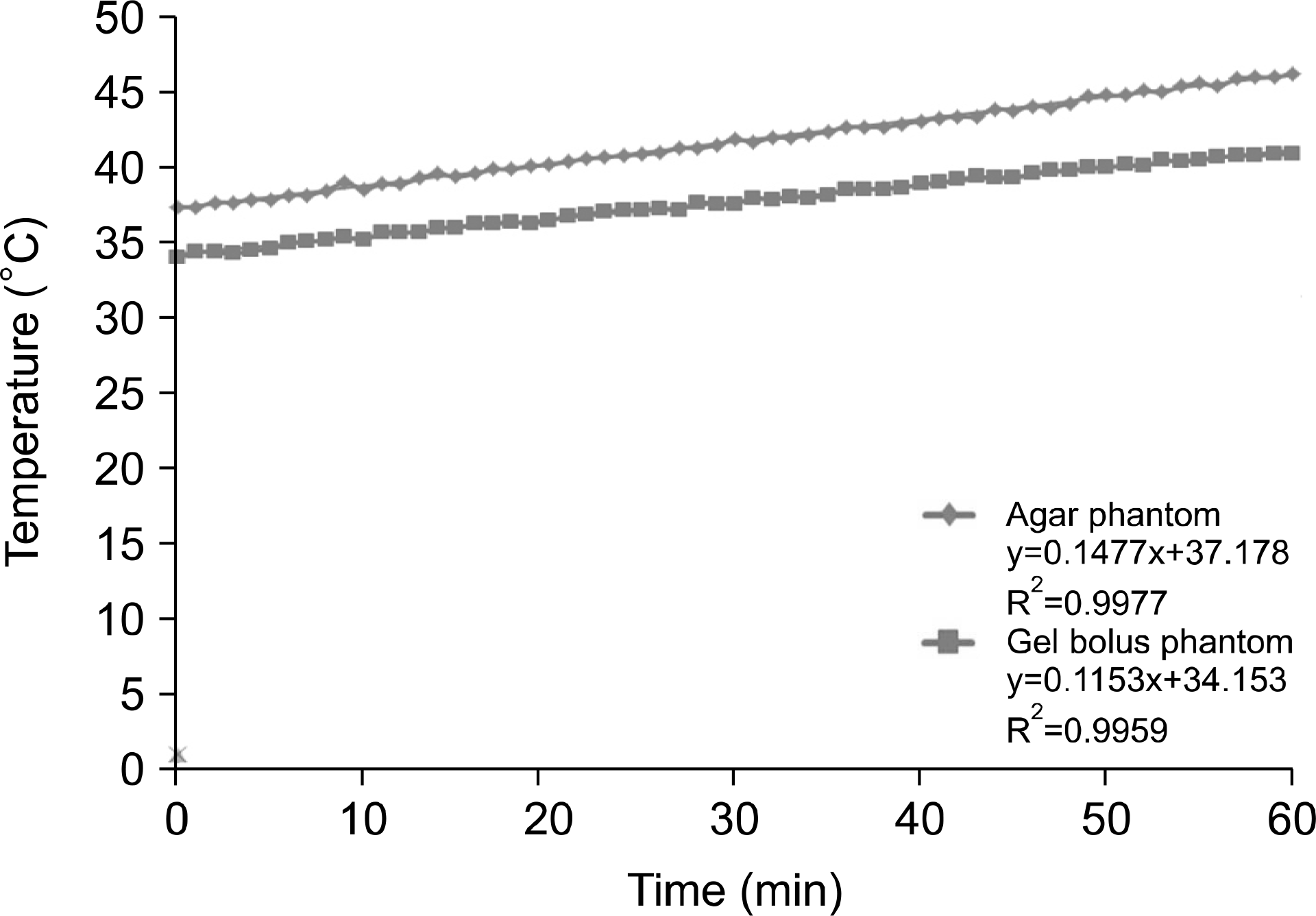
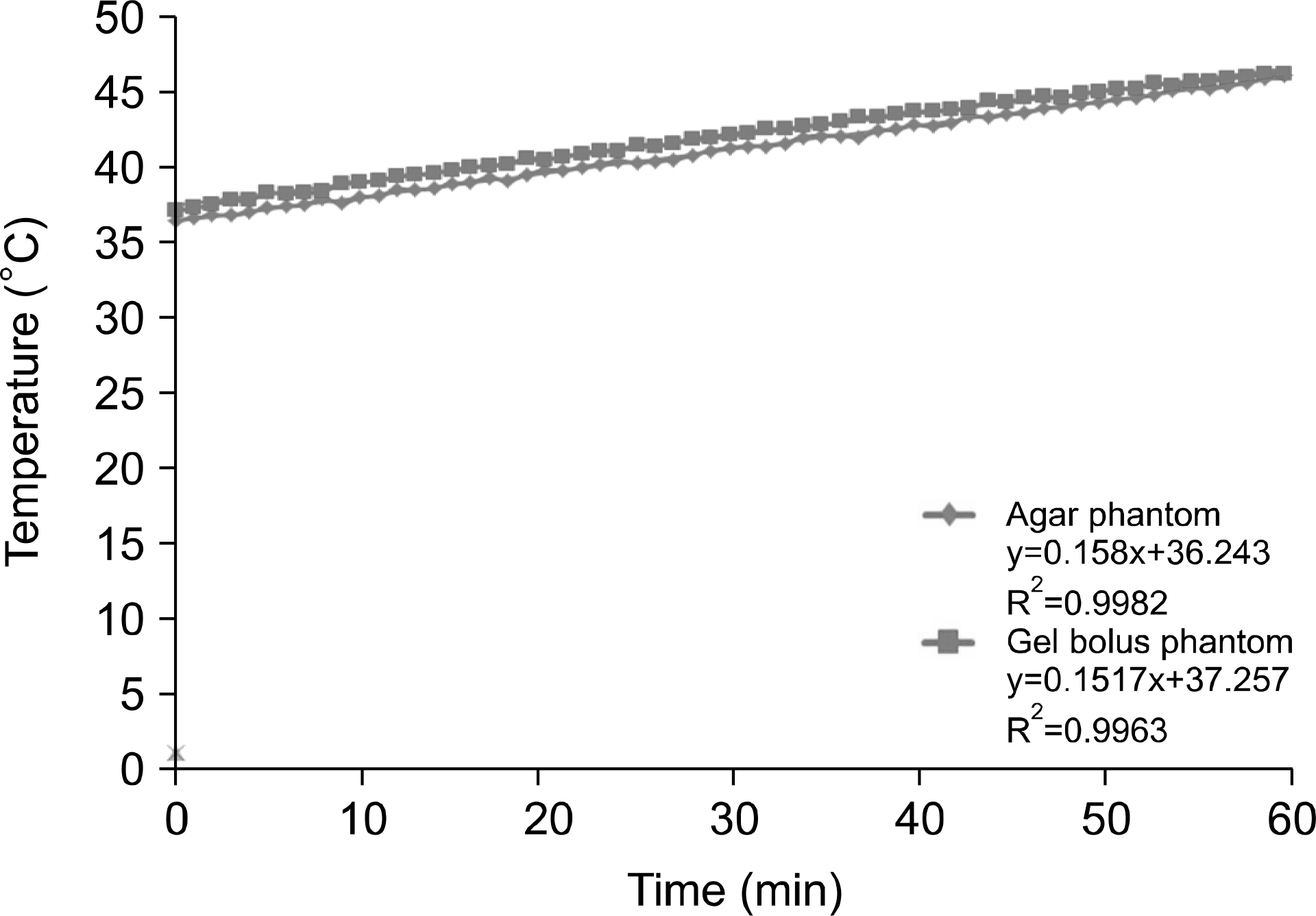 | Fig. 10.5-cm depth for 60 min at 150 W output power for the temperature analysis of the agar and gel bolus phantoms. |
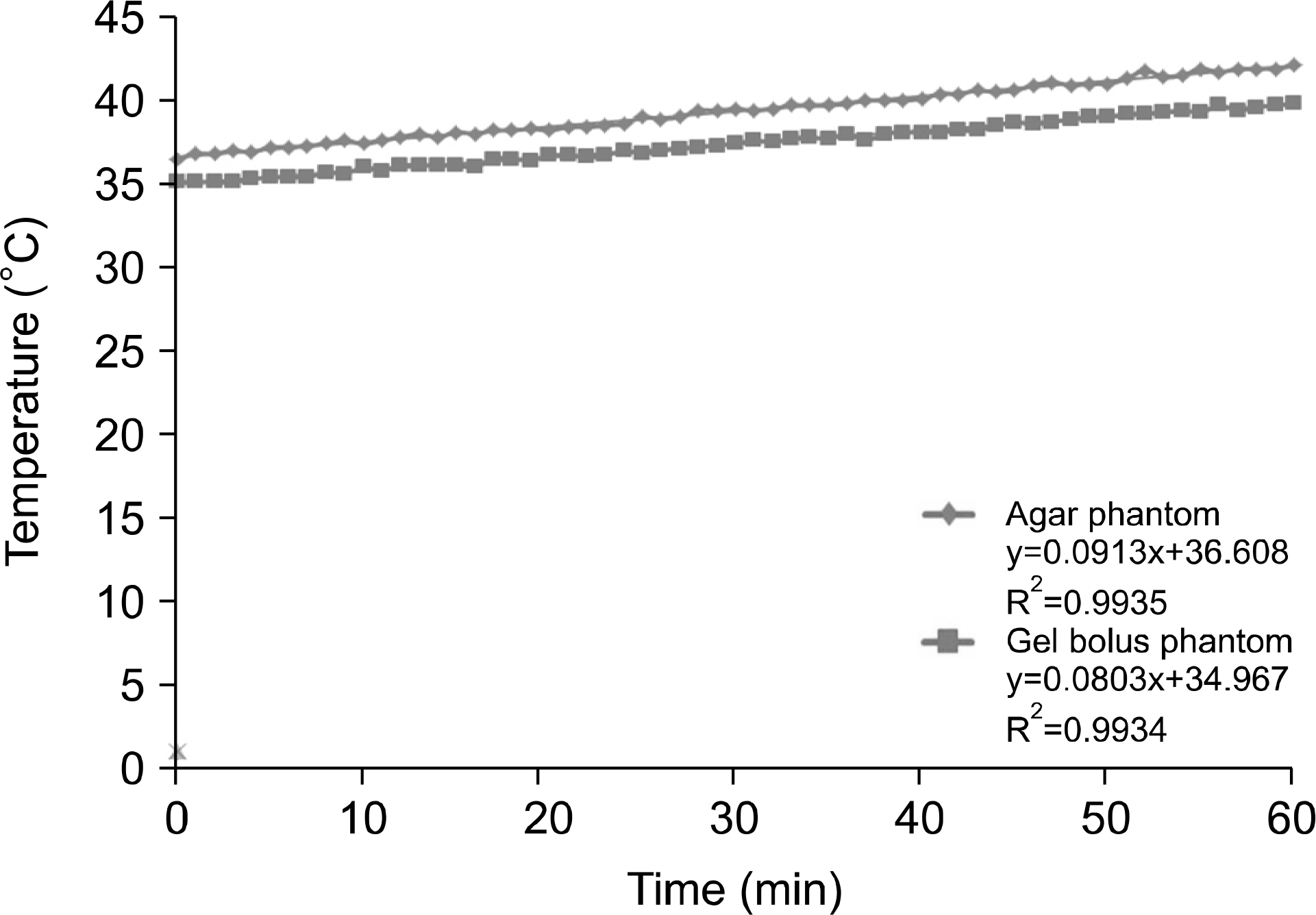 | Fig. 11.10-cm depth for 60 min at 100 W output power for the temperature analysis of the agar and gel bolus phantoms. |
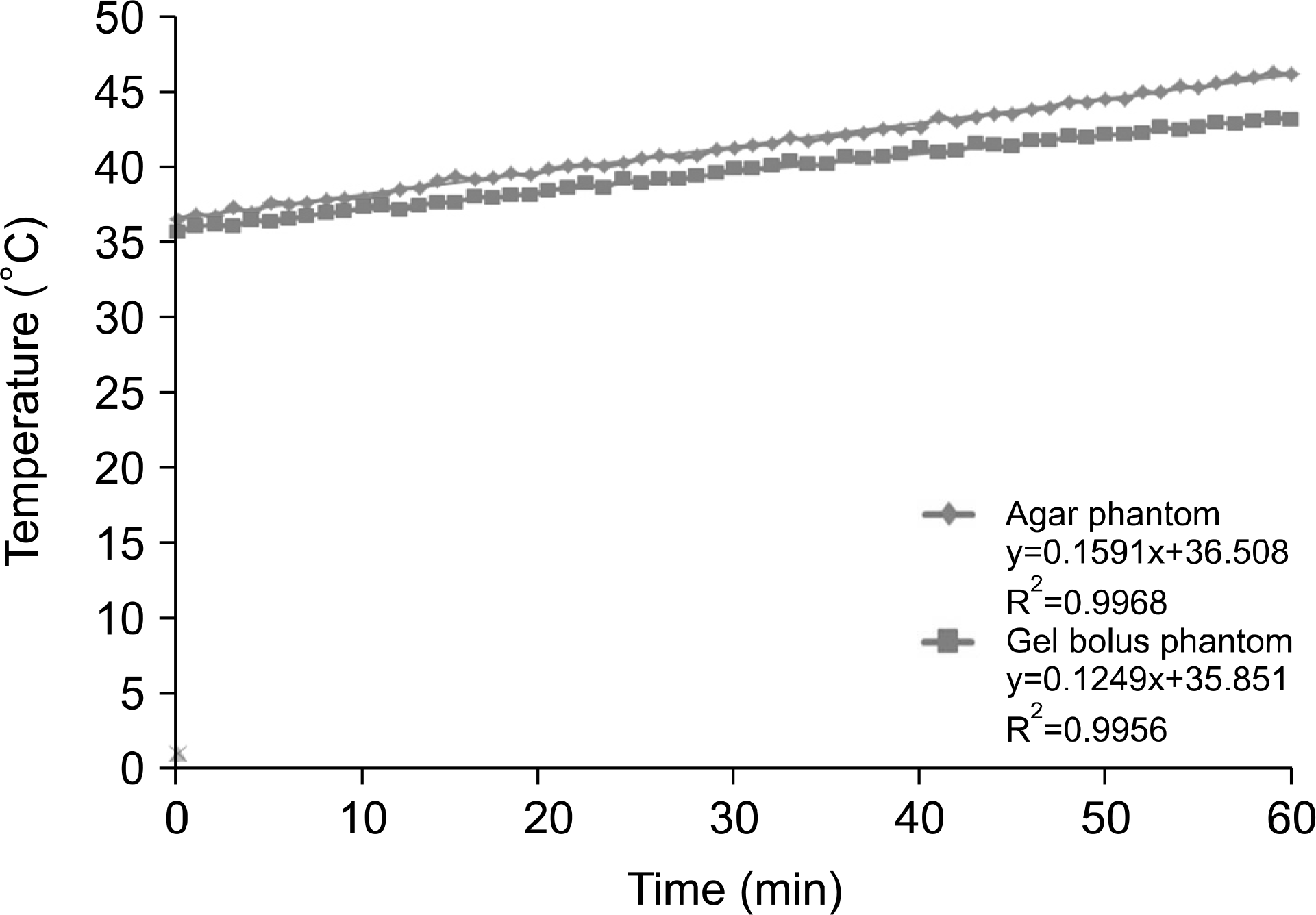 | Fig. 12.10-cm depth for 60 min at 150 W output power for the temperature analysis of the agar and gel bolus phantoms. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download