Abstract
The purpose of this study is to evaluate efficacy and feasibility of adaptive radiotherapy according to tumor volume change (TVC) in early stage non-small cell lung cancer (NSCLC) using stereotactic body radiotherapy (SBRT). Twenty-two lesions previously treated with SBRT were selected. SBRT was usually performed with a total dose of 48 Gy or 60 Gy in four fractions with an interval of three to four days between treatments. For evaluation of TVC, gross tumor volume (GTV) was contoured on each cone-beam computed tomography (CBCT) image used for image guidance. Intensity modulated radiotherapy (IMRT) planning was performed in the first CBCT (CBCT1) using a baseline plan. For ART planning (ART), re-optimization was performed at 2nd, 3rd, and 4th CBCTs (CBCT2, CBCT3, and CBCT4) using the same angle and constraint used for the baseline plan. The ART plan was compared with the non-ART plan, which generated copying of the baseline plan to other CBCTs. Average GTV volume was 10.7 cc. Average TVC was −1.5%, 7.3%, and −25.1% in CBCT2, CBCT3, and CBCT4 and the TVC after CBCT3 was significant (p<0.05). However, the nine lesions were increased GTV in CBCT2. In the ART plan, V20 Gy, D1500 cc, and D1000 cc of lung were significantly decreased (p<0.05), and V30 Gy and V32 Gy of the chest wall were also decreased (p<0.05). While D min of planning target volume (PTV) decreased by 8.3% in the non-ART plan of CBCT2 compared with the baseline plan in lesions with increased tumor size (p=0.021), PTV coverage was not compromised in the ART plan. Based on this result, use of the ART plan may improve target coverage and OAR saving. Thus ART using CBCT should be considered in early stage NSCLC with SBRT.
Go to : 
References
1. Ricardi U, Frezza G, Filippi AR, et al. Stereotactic Ablative Radiotherapy for stage I histologically proven non-small cell lung cancer: an Italian multicenter observational study. Lung cancer. 84(3):248–53. 2014.

2. Song SY, Choi W, Shin SS, et al. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung cancer. 66(1):89–93. 2009.

3. Matsuo Y, Chen F, Hamaji M, et al. Comparison of longterm survival outcomes between stereotactic body radiotherapy and sublobar resection for stage I non-small-cell lung cancer in patients at high risk for lobectomy: A propensity score matching analysis. European journal of cancer. 50(17):2932–8. 2014.

4. Port JL, Parashar B, Osakwe N, et al. A propensity matched analysis of wedge resection and stereotactic body radiotherapy for early stage lung cancer. The Annals of thoracic surgery. 98(4):1152–9. 2014.
5. Zhang B, Zhu F, Ma X, et al. Matched-pair comparisons of stereotactic body radiotherapy (SBRT) versus surgery for the treatment of early stage non-small cell lung cancer: a systematic review and metaanalysis. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 112(2):250–5. 2014.

6. Kestin L, Grills I, Guckenberger M, et al. Dose-response relationship with clinical outcome for lung stereotactic body radiotherapy (SBRT) delivered via online image guidance. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 110(3):499–504. 2014.

7. Koshy M, Malik R, Weichselbaum RR, Sher DJ. Increasing radiation therapy dose is associated with improved survival in patients undergoing stereotactic body radiation therapy for stage I non-small-cell lung cancer. International journal of radiation oncology, biology, physics. 91(2):344–50. 2015.
8. Matsuo Y, Shibuya K, Nakamura M, et al. Dosevolume metrics associated with radiation pneumonitis after stereotacticbody radiation therapy for lung cancer. International journal of radiation oncology, biology, physics. 83(4):e545–9. 2012.
9. Asai K, Shioyama Y, Nakamura K, et al. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy: risk factors and dose-volume relationship. International journal of radiation oncology, biology, physics. 84(3):768–73. 2012.

10. Dunlap NE, Cai J, Biedermann GB, et al. Chest wall volume receiving >30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. International journal of radiation oncology, biology, physics. 76(3):796–801. 2010.

11. Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 24(30):4833–9. 2006.

12. Erridge SC, Seppenwoolde Y, Muller SH, et al. Portal imaging to assess setup errors, tumor motion and tumor shrinkage during conformal radiotherapy of non-small cell lung cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 66(1):75–85. 2003.

13. Siker ML, Tome WA, Mehta MP. Tumor volume changes on serial imaging with megavoltage CT for non-small-cell lung cancer during intensitymodulated radiotherapy: how reliable, consistent, and meaningful is the effect? International journal of radiation oncology, biology, physics. 66(1):135–41. 2006.

14. Bhatt AD, El-Ghamry MN, Dunlap NE, et al. Tumor volume change with stereotactic body radiotherapy (SBRT) for early-stage lung cancer: evaluating the potential for adaptive SBRT. American journal of clinical oncology. 38(1):41–6. 2015.
15. Gunter T, Ali I, Matthiesen C, et al. Gross tumour volume variations in primary non-small-cell lung cancer during the course of treatment with stereotactic body radiation therapy. Journal of medical imaging and radiation oncology. 58(3):384–91. 2014.

16. Saito AI, Olivier KR, Li JG, et al. Lung tumor motion change during stereotactic body radiotherapy (SBRT): an evaluation using MRI. Journal of applied clinical medical physics/American College of Medical Physics. 15(3):4434. 2014.

17. Tatekawa K, Iwata H, Kawaguchi T, et al. Changes in volume of stage I non-small-cell lung cancer during stereotactic body radiotherapy. Radiation oncology. 9:8. 2014.

18. Yi BS, Perks J, Houston R, et al. Changes in position and volume of lung cancer target volumes during stereotactic body radiotherapy (SBRT): is image guidance necessary? Technology in cancer research & treatment. 10(5):495–504. 2011.

19. Feng M, Kong FM, Gross M, et al. Using fluorodeoxyglucose positron emission tomography to assess tumor volume during radiotherapy for non-small-cell lung cancer and its potential impact on adaptive dose escalation and normal tissue sparing. International journal of radiation oncology, biology, physics. 73(4):1228–34. 2009.

20. Bral S, Duchateau M, De Ridder M, et al. Volumetric response analysis during chemoradiation as predictive tool for optimizing treatment strategy in locally advanced unresectable NSCLC. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 91(3):438–42. 2009.

21. Britton KR, Starkschall G, Tucker SL, et al. Assessment of gross tumor volume regression and motion changes during radiotherapy for non-small-cell lung cancer as measured by four-dimensional computed tomography. International journal of radiation oncology, biology, physics. 68(4):1036–46. 2007.

22. Kupelian PA, Ramsey C, Meeks SL, et al. Serial megavoltage CT imaging during external beam radiotherapy for non-small-cell lung cancer: observations on tumor regression during treatment. International journal of radiation oncology, biology, physics. 63(4):1024–8. 2005.

23. Westhoff PG, de Graeff A, Geerling JI, Reyners AK, van der Linden YM. Dexamethasone for the prevention of a pain flare after palliative radiotherapy for painful bone metastases: a multicenter double-blind placebocontrolled randomized trial. BMC cancer. 14:347. 2014.

24. Pan HY, Allen PK, Wang XS, et al. Incidence and predictive factors of pain flare after spine stereotactic body radiation therapy: secondary analysis of phase 1/2 trials. International journal of radiation oncology, biology, physics. 90(4):870–6. 2014.

25. Shirata Y, Jingu K, Koto M, et al. Prognostic factors for local control of stage I non-small cell lung cancer in stereotactic radiotherapy: a retrospective analysis. Radiation oncology. 7:182. 2012.

26. Barriger RB, Forquer JA, Brabham JG, et al. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. International journal of radiation oncology, biology, physics. 82(1):457–62. 2012.

27. Yoo S, Yin FF. Dosimetric feasibility of cone-beam CT-based treatment planning compared to CT-based treatment planning. International journal of radiation oncology, biology, physics. 66(5):1553–61. 2006.

28. Yang Y, Schreibmann E, Li T, Wang C, Xing L. Evaluation of on-board kV cone beam CT (CBCT)-based dose calculation. Physics in medicine and biology. 52(3):685–705. 2007.

29. Altorjai G, Fotina I, Lutgendorf-Caucig C, et al. Cone-beam CT-based delineation of stereotactic lung targets: the influence of image modality and target size on interobserver variability. International journal of radiation oncology, biology, physics. 82(2):e265–72. 2012.

30. Guckenberger M, Richter A, Wilbert J, Flentje M, Partridge M. Adaptive radiotherapy for locally advanced non-small-cell lung cancer does not underdose the microscopic disease and has the potential to increase tumor control. International journal of radiation oncology, biology, physics. 81(4):e275–82. 2011.

Go to : 
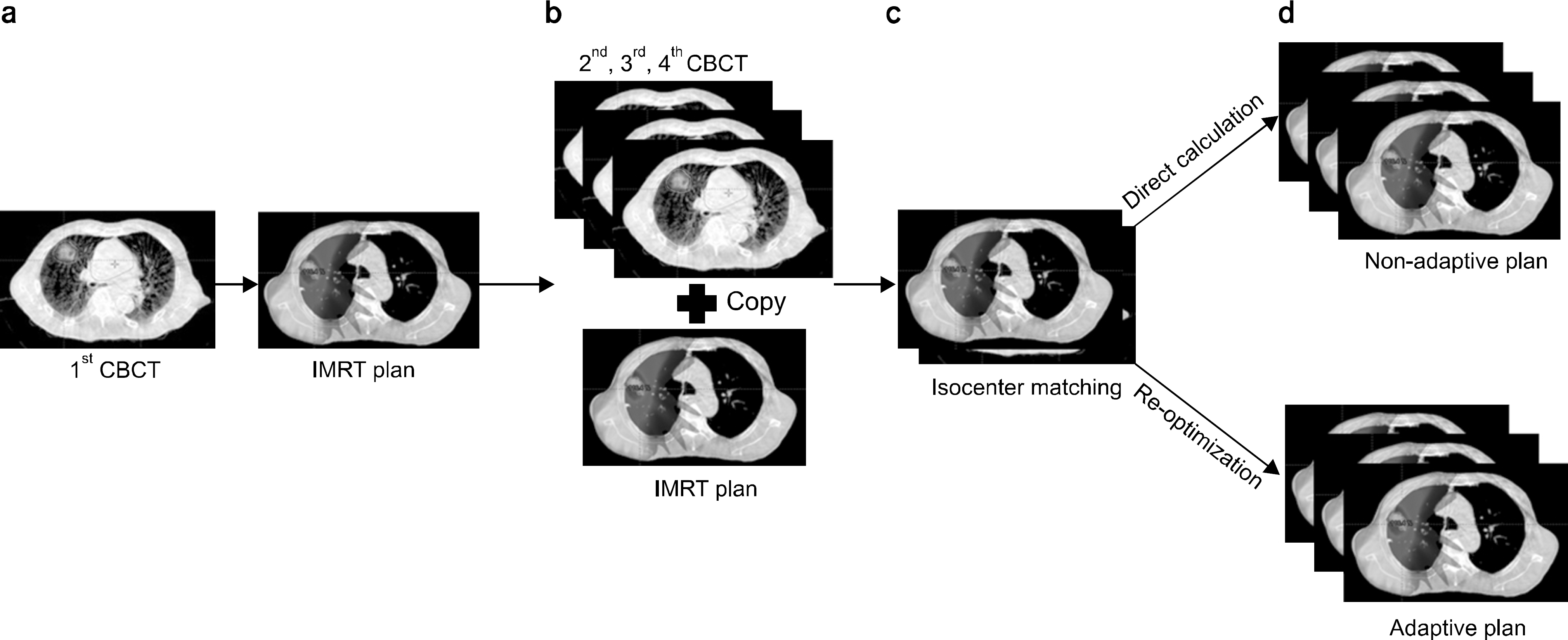 | Fig. 1.Schematics of the planning procedure. (a) The intensity modulated radiotherapy (IMRT) plan was performed in 1st CBCT (baseline plan). (b) The beam angle and MLC motion of baseline plan was copied to other CBCTs. (c) The treatment isocenter was adjusted based on 3-dimensional registration data of each CBCT using the Varian offline review program (Varian Medical Systems Inc., Palo Alto, CA). (d) Non-adaptive plans were generated by direct calculation. Adaptive plans were generated by re-optimization process. |
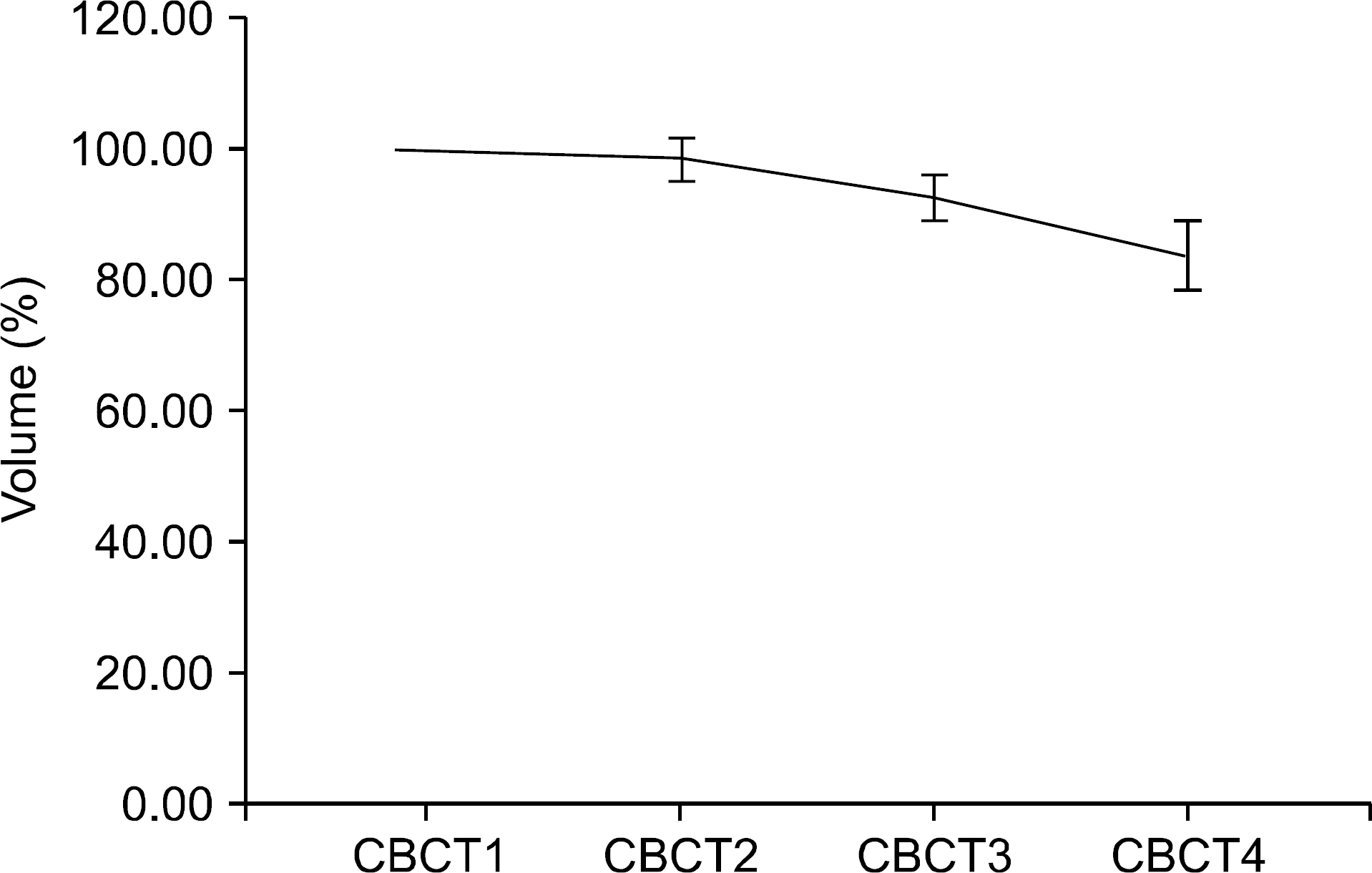
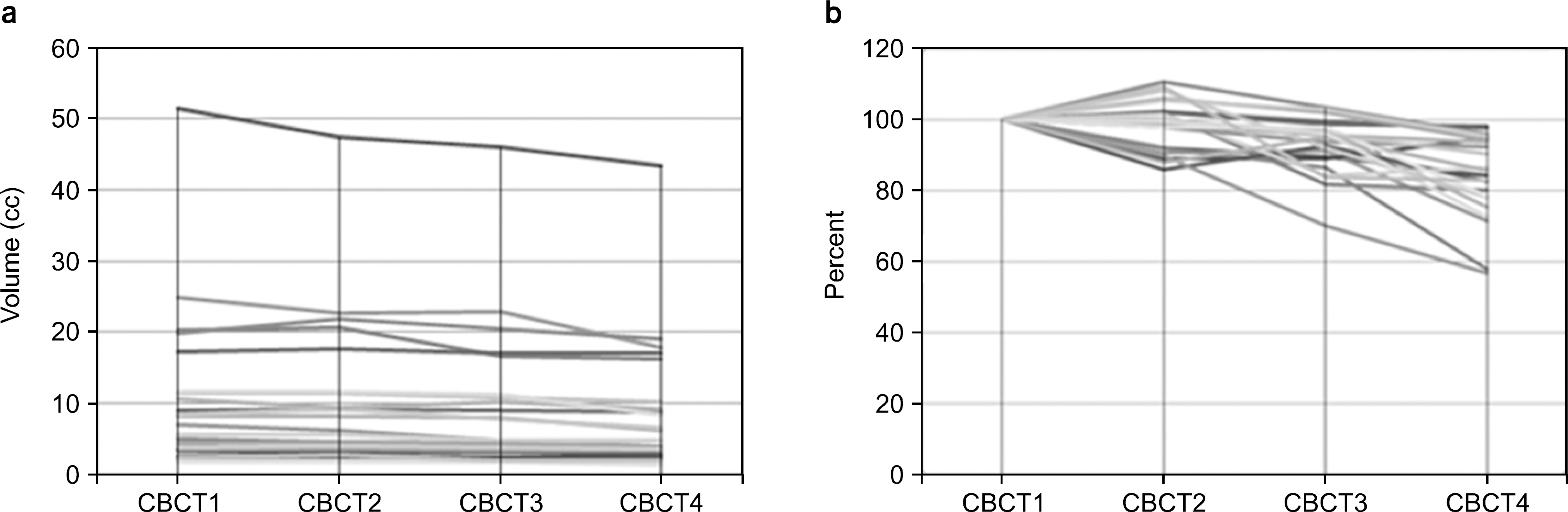 | Fig. 2.The absolute and relative volume of GTV in all studied patients. (a) absolute GTV. (b) relative GTV. |
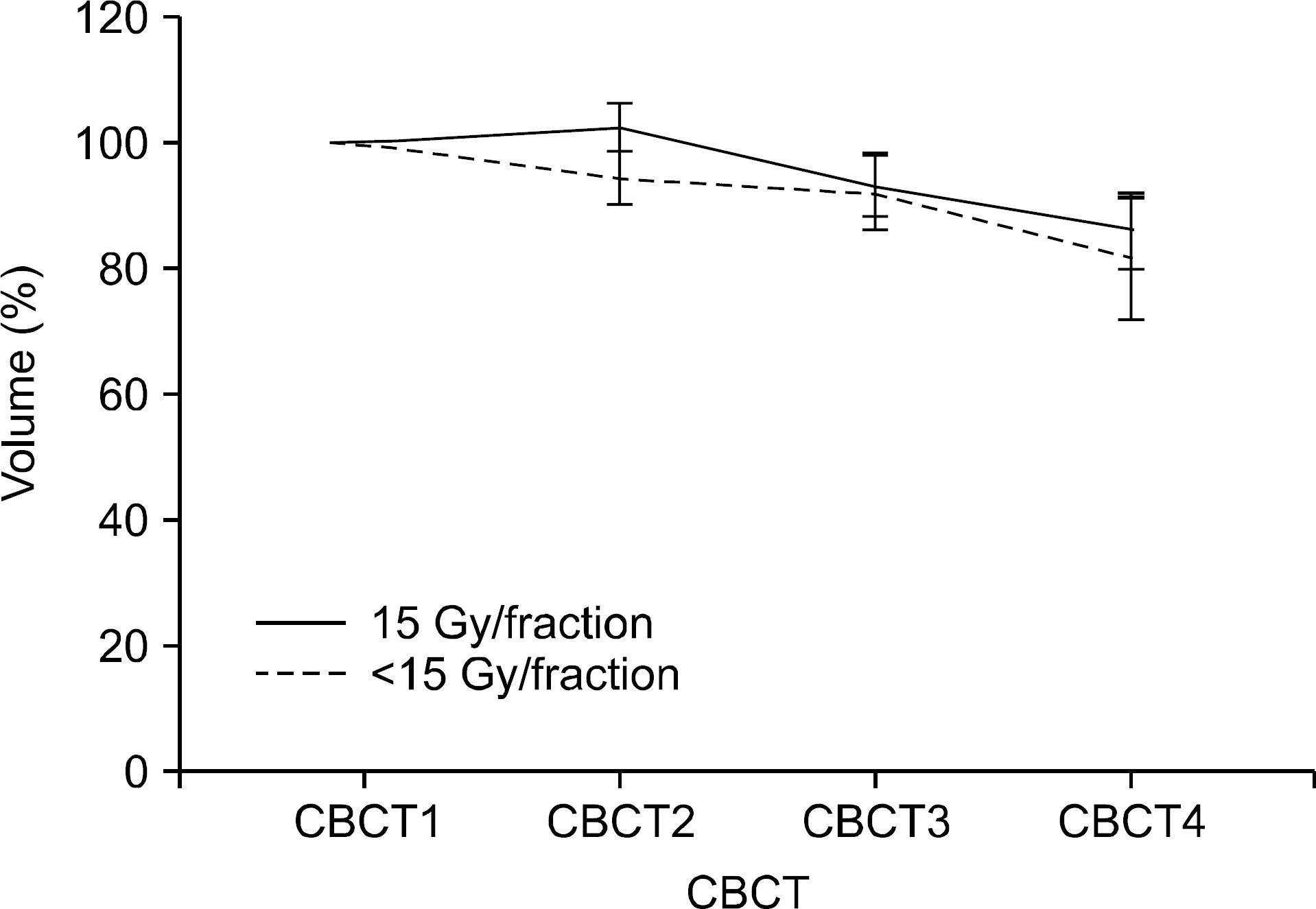 | Fig. 4.Differences of GTV change according to dose schedule. GTV in lesions treated with 15 Gy per fraction was significantly larger than the volume of tumors treated with less than 15 Gy per fraction (p=0.005). |
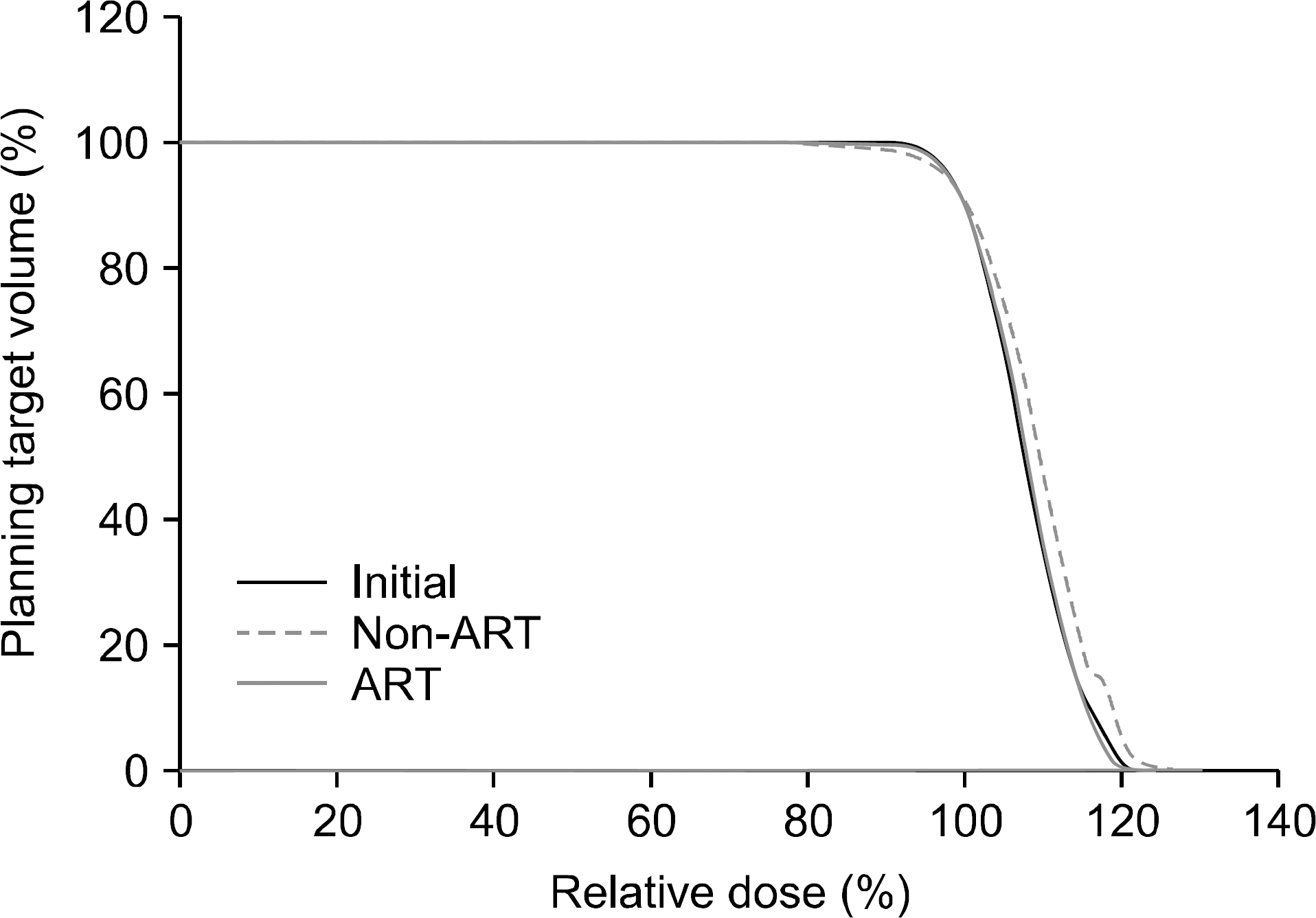 | Fig. 5.The dose-volume histogram of PTV coverage on the baseline plan in CBCT1, non-adaptive plan and adaptive plan of CBCT2. While Dmin was decreased by approximately 8.3% in non-ART (p<0.021), PTV coverage was not compromised compared with the initial plan in the ART plan. |
Table 1.
Absolute and relative volume of GTV in the course of stereotactic body radiotherapy.
Table 2.
Dosimetric parameters comparing non-adaptive versus adaptive plan.
Table 3.
Comparisons of PTV coverage of non-adaptive and adaptive plans in CBCT2 compared with CBCT1.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download