Abstract
Organic solvents are known toxic effects like vertigo, behavioral obstacle, distracting, and peripheral neuropathy in neuron areas. However, there have been few studies how neurotoxic solvents-exposed workers are affected by the cognitive load of preceding working memory tasks. Therefore, we used fMRI as to measure the neural correlates of working memory impairment in occupational workers who had from chronic exposure to organic solvent. Twenty-nine solvent-exposed workers were included in this study. Each participant concluded the verbal N-back tasks (1- and 2-back) during the fMRI acquisition. Within-group analyses showed fronto-parietal networks were active in each condition. Direct comparisons between 1- and 2-back showed higher activation during the 2-back than 1-back. We found that increased activation of these regions at lower task demand is associated with increased cost of implementing.
Go to : 
References
1. Bale AS, Barone S, Jr. , Scott CS, Cooper GS. A review of potential neurotoxic mechanisms among three chlorinated organic solvents. Toxicol Appl Pharmacol. 255:113–126. 2011.

2. Spencer PS, Schaumburg HH, Sabri MI, Veronesi B. The enlarging view of hexacarbon neurotoxicity. Crit Rev Toxicol. 7:279–356. 1980.

3. Firestone JA, Longstrength WTJ. Neurologic and psychiatric disorders. Rosenstock L, Cullen M, Brodkin C, Redlich C: Textbook of clinical occupational and environmental medicine. 4thed, Saunders Elsevier, Philadelphia (. 2004. ), pp.645–660.
5. Baker EL. A review of recent research on health effects of human occupational exposure to organic solvents. A critical review. J Occup med. 36:1079–1092. 1994.
6. Haut MW, Leach S, Kuwabara H, et al. Verbal working memory and solvent exposure: a positron emission tomography study. Neuropsychology. 14:551–558. 2000.

7. Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a metaanalysis of normative functional neuroimaging studies. Hum Brain Mapp. 25:46–59. 2005.

8. Evans A, Collins DL, Mills SR, et al. 3D statistical neuroanatomical models from 305 MRI volumes. Proc IEEE-Nuclear Science Symposium and Medical Imaging Conference. 3:1813–1817. 1993.

9. Braver TS, Cohen JD, Nystrom LE, et al. A parametric study of prefrontal cortex involvement in human working memory. NeuroImage. 5:49–62. 1997.

10. Cohen JD, Perlstein WM, Braver TS, et al. Temporal dynamics of brain activation during a working memory task. Nature. 386:604–608. 1997.

11. Petrides M. Dissociable roles of mid-dorsolateral prefrontal and anterior inferotemporal cortex in visual working memory. Journal of Neuroscience. 20(19):7496–7503. 2000.

12. Hagoort P, Hald L, Bastiaansen M, Petersson KM. Integration of word meaning and world knowledge in language comprehension. Science. 304(5669):438–441. 2004.

13. Barbey AK, Kruger F, Grafman J. An evolutionarily adaptive neural architecture for social reasoning. Trends Neurosci. 32(12):603–610. 2009.

14. Rypma B, Berger JS, D'Esposito M. The influence of working memory demand and subject performance on prefrontal cortical activity. J CognNeurosci. 14(5):721–731. 2002.
15. Davachi L, Maril A, Wagner AD. When keeping in mind supports later bringing to mind: neural markers of phonological rehearsal predict subsequent remembering. Journal of Cognitive Neuroscience. 13(8):1059–1070. 2001.

Go to : 
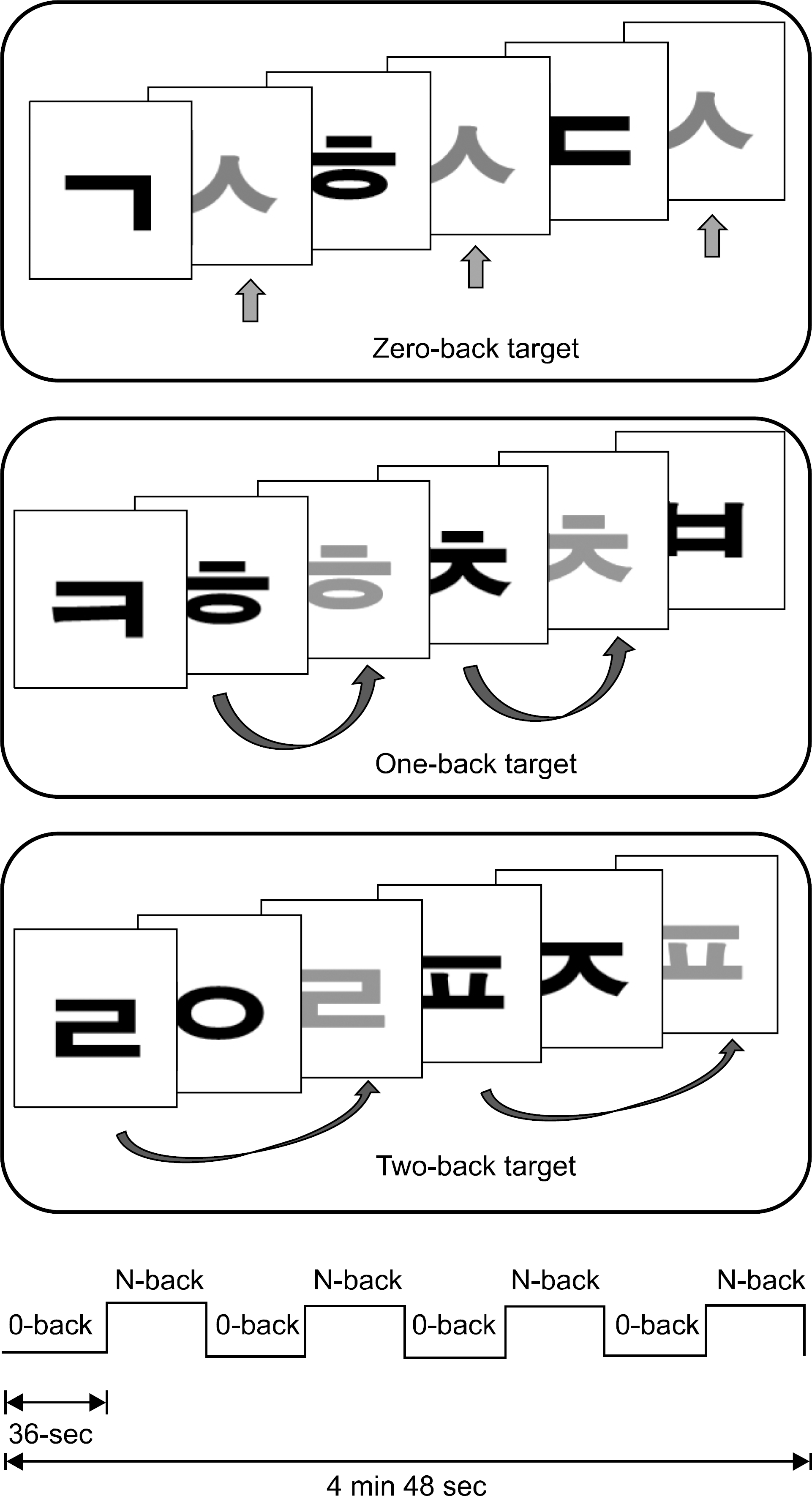 | Fig. 1.N-back task paradigm and schematic of block design. In the 0-back condition, participants were asked to identify the target letter “ㅅ” that was presented at the beginning of each trial block. In the N-back (N is 1 or 2) condition, they had to permanently memorize the recently presented letters in order to press a button whent a letter matched one that had been presented N letters before the present letter. The entire functional scanning run took approximately 4 min 48 sec. |
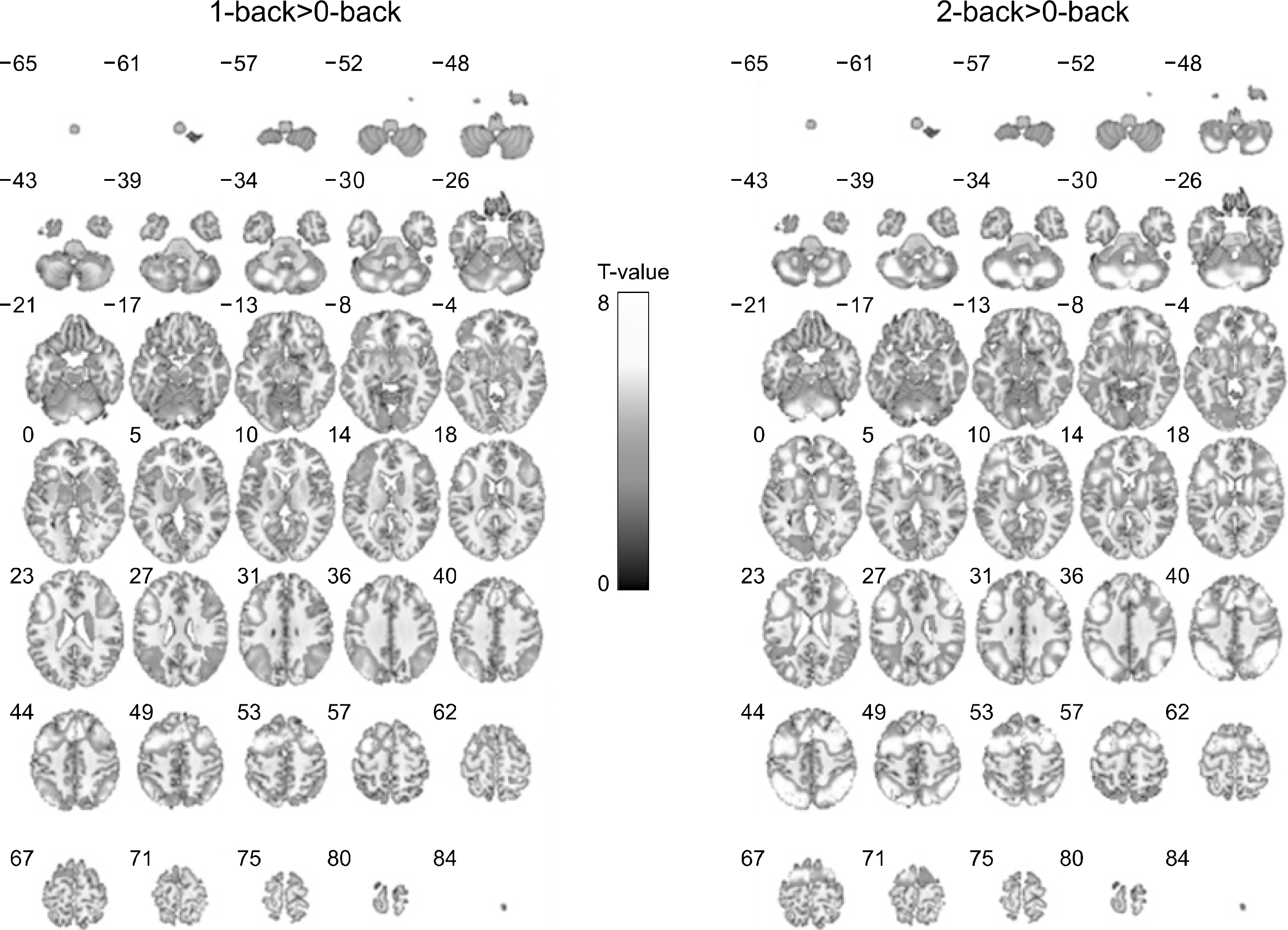 | Fig. 2.Brain activation maps contrasted from 1- or 2-back minus 0-back task for all organic solvent-exposed subjects. The N-back working memory tasks revealted that a network of frontal and parietal cortical areas was active in both two conditions. |
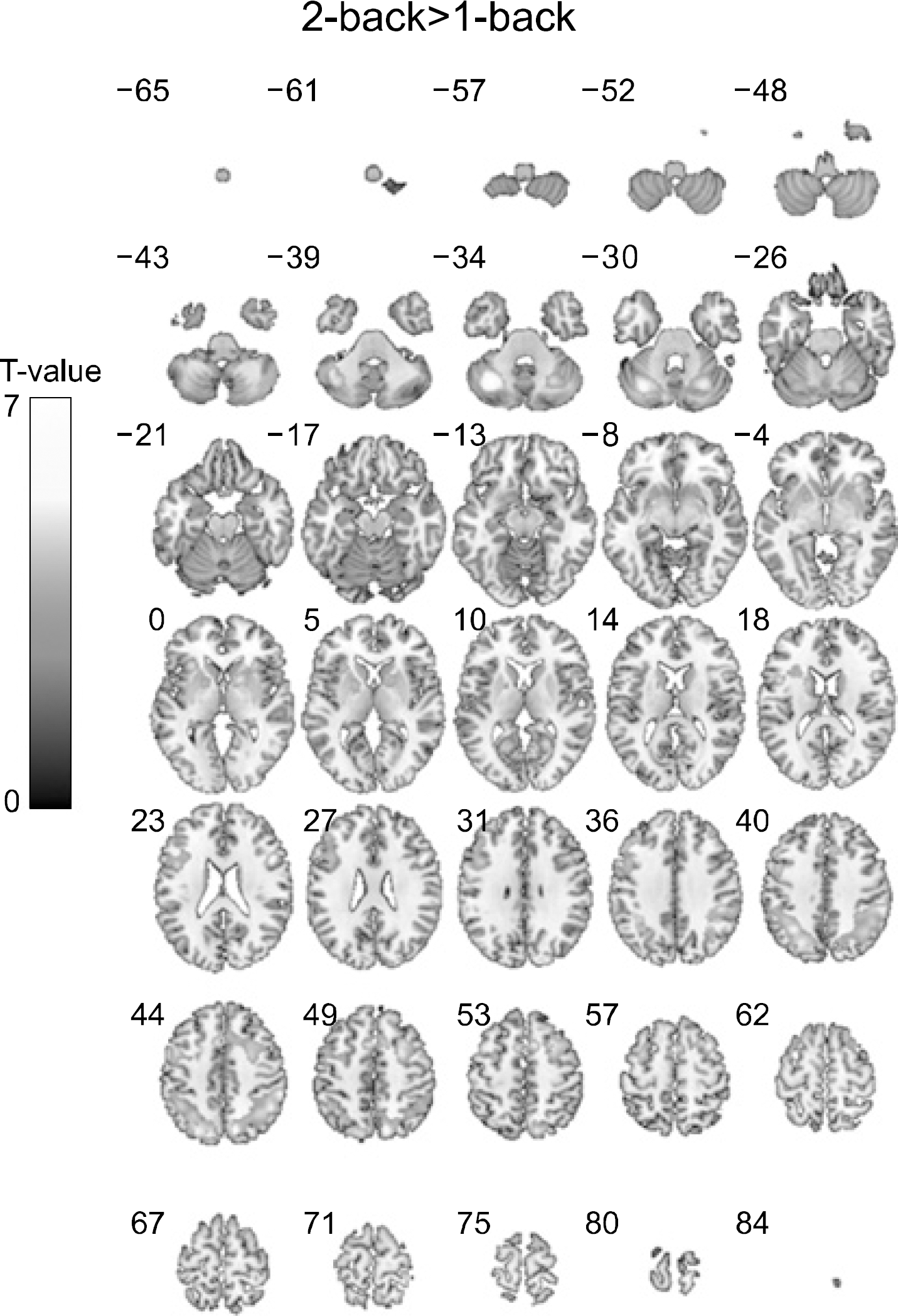 | Fig. 3.Brain activation maps derived from direct comparison between 2-back task and 1-back task. During the 2-back task, the organic solvent-exposed subjects showed higher activation than performing the 1-back task in the frontal and parietal cortical areas. No region showed significantly higher activation in the performing the 1-back task compared to the 2-back task. |
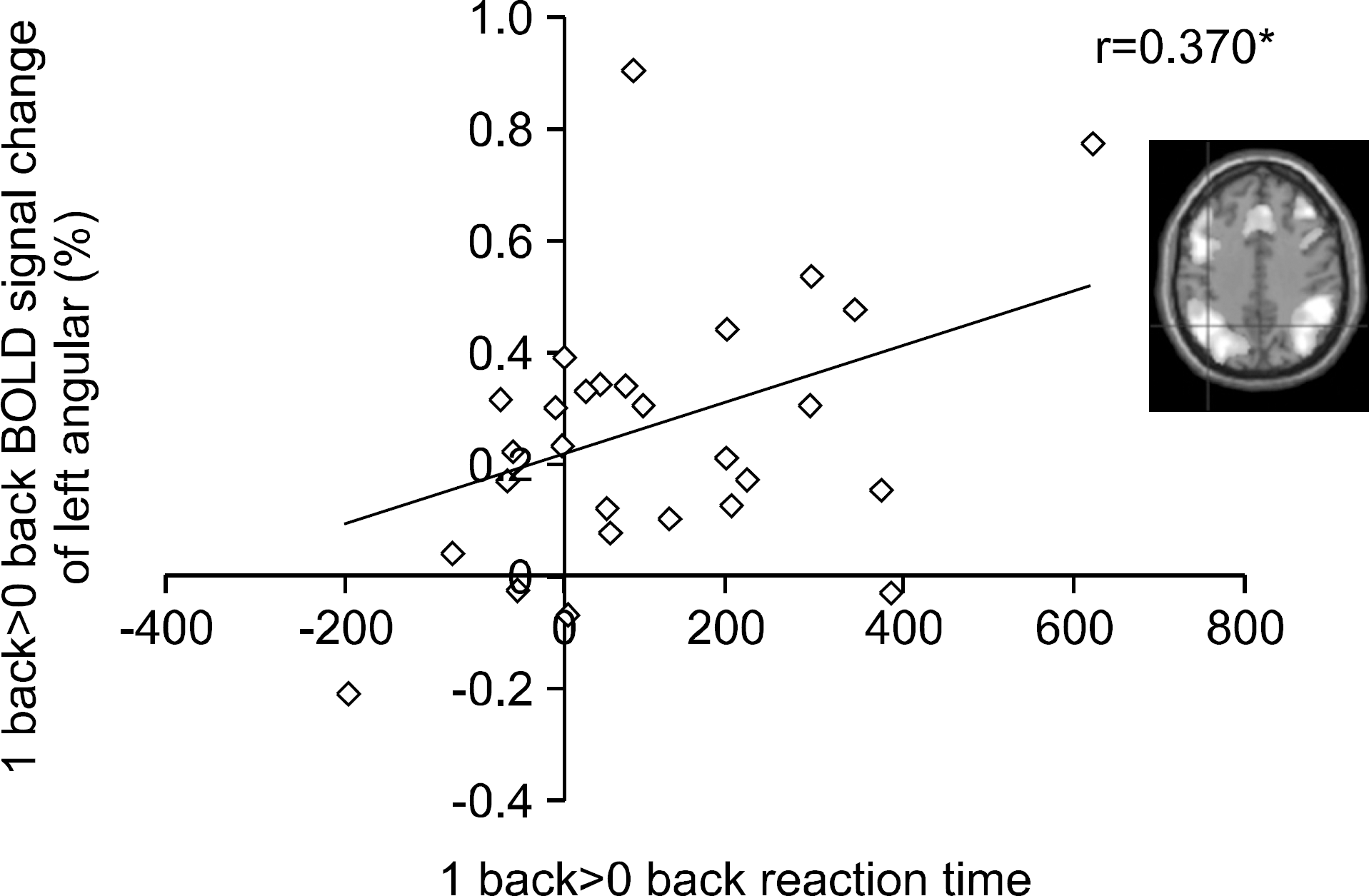 | Fig. 4.Correlation between BOLD signal change and 1-back task performance (reaction time) (R=0.370, p<0.05). |
Table 1.
Demographic, clinical, and laboratory characteris tics of organic solvent-exposed subjects.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download