Abstract
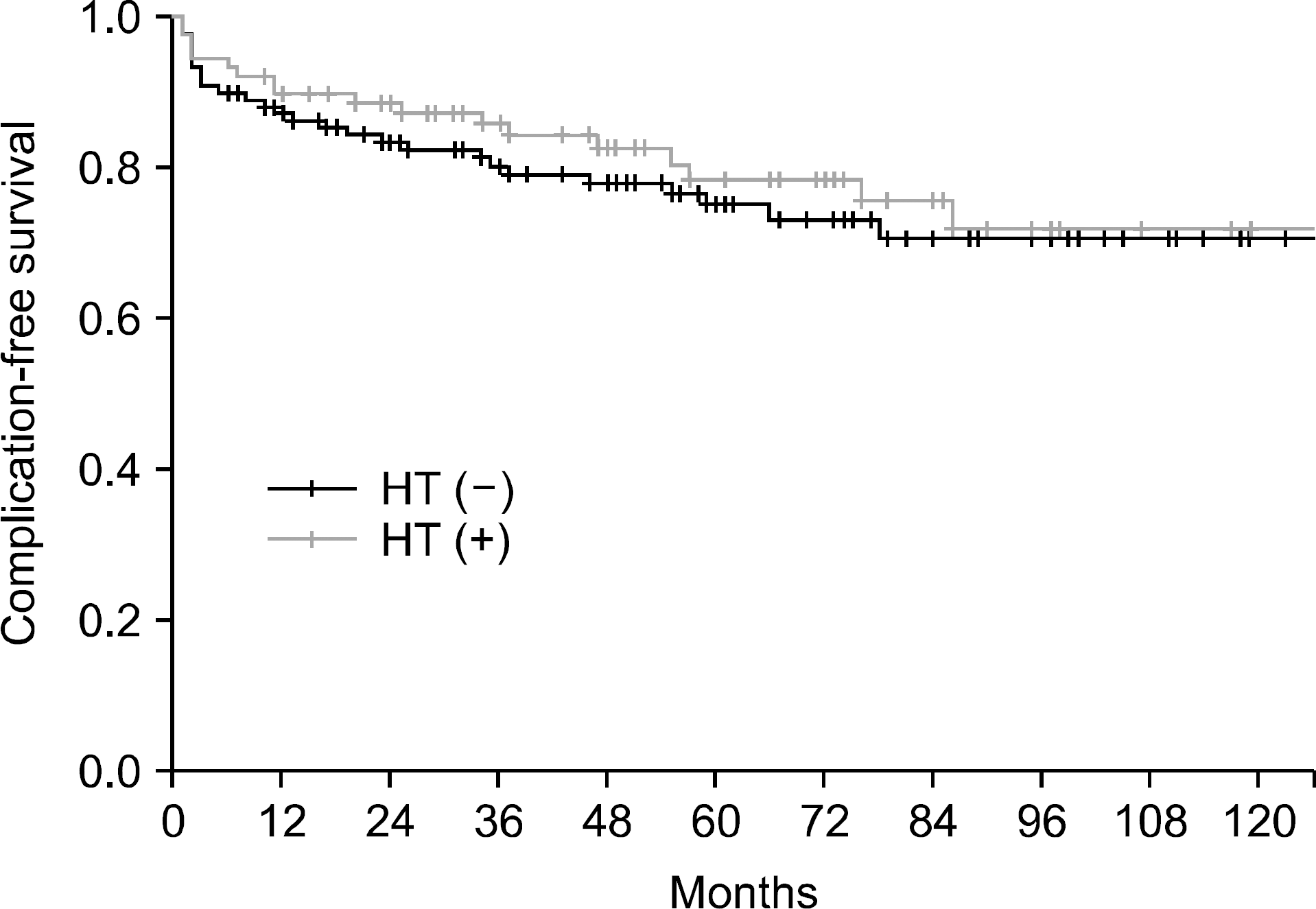
We investigated whether regional hyperthermia (HT) increased post-surgical complications in patients with locally advanced rectal cancer treated with preoperative concurrent chemoradiotherapy (CCRT). Between 1996 and 2007, 205 patients treated with preoperative CCRT and curative surgery were evaluable for the analysis of acute and late toxicities. A total dose of 39.6 Gy or 45 Gy was delivered concurrently with one or two cycles of chemotherapy (5-fluorouracil, leucovorin). Eighty-eight patients received regional HT twice a week using an 8-MHz radiofrequency capacitive heating device. Surgery was performed 4∼6 weeks after the completion of preoperative CCRT. The median age was 59 years (range, 18∼83) and the median follow-up period was 61months (range, 2∼191). The 5-year overall survival and complication-free survival rate of all patients was 77.4% and 73.7%, respectively. Early leakage, delayed leakage, anastomotic stricture, fistula, and small bowel obstruction occurred in 1.0%, 2.9%, 1.5%, 5.9%, and 17.1%, respectively. HT did not increase all kinds of complications. The 5-year complication-free survival rate was 71.8% in the non-HT group and 76.3% in the HT group (p=0.293). Regional HT did not increase postoperative complications in patients with locally advanced rectal cancer treated with preoperative CCRT followed by curative surgery.
Go to : 
References
1. McCall JL, Cox MR, Wattchow DA. Analysis of local recurrence rates after surgery alone for rectal cancer. Int J Colorectal Dis. 10:126–32. 1995.

2. NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 264:1444–1450. 1990.
3. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 351:1731–1740. 2004.

4. Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol). 19:418–26. 2007.

5. De Haas-Kock DF, Buijsen J, Pijls-Johannesma M, et al. Concomitant hyperthermia and radiation therapy for treating locally advanced rectal cancer. Cochrane Database Syst Rev. 2009. CD006269.

6. Kang MK, Kim MS, Kim JH. Clinical outcomes of mild hyperthermia for locally advanced rectal cancer treated with preoperative radiochemotherapy. Int J Hyperthermia. 27:482–490. 2011.

7. Berdov BA, Menteshashvili GZ. Thermoradiotherapy of patients with locally advanced carcinoma of the rectum. Int J Hyperthermia. 6:881–890. 1990.

8. van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 355:1119–1125. 2000.
9. Maluta S, Romano M, Dall'oglio S, et al. Regional hyperthermia added to intensified preoperative chemoradiation in locally advanced adenocarcinoma of middle and lower rectum. Int J Hyperthermia. 26:108–117. 2010.

10. Anscher MS, Lee C, Hurwitz H, et al. A pilot study of preoperative continuous infusion 5-fluorouracil, external microwave hyperthermia, and external beam radiotherapy for treatment of locally advanced, unresectable, or recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 47:719–24. 2000.

11. Wust P, Rau B, Gellerman J, et al. Radiochemotherapy and hyperthermia in the treatment of rectal cancer. Recent ResultsCancer Res. 146:175–191. 1998.

12. Rau B, Wust P, Hohenberger P, et al. Preoperative hyperthermia combined with radiochemotherapy in locally advanced rectal cancer: a phase II clinical trial. Ann Surg. 227:380–389. 1998.
13. Ceelen WP, Van Nieuwenhove Y, Fierens K. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev. 2009. CD006041.

14. Schulze T, Wust P, Gellermann J, et al. Influence of neoadjuvant radiochemotherapy combined with hyperthermia on the quality of life in rectum cancer patients. Int J Hyperthermia. 22:301–318. 2006.

15. Rullier E, Laurent C, Garrelon JL, Michel P, Saric J, Parneix M. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg. 85:355–358. 1998.

16. Vignali A, Fazio VW, Lavery IC, et al. Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1,014 patients. J Am Coll Surg. 185:105–113. 1997.

17. Yeh CY, Changchien CR, Wang JY, et al. Pelvic drainage and other risk factors for leakage after elective anterior resection in rectal cancer patients: a prospective study of 978 patients. Ann Surg. 241:9–13. 2005.
18. Kim CW, Kim JH, Yu CS, et al. Complications after sphincter-saving resection in rectal cancer patients according to whether chemoradiotherapy is performed before or after surgery. Int J Radiat Oncol Biol Phys. 78:156–163. 2010.

19. Horie H, Kashiwagi H, Konishi F, Furuta K, Ozawa A, Kanazawa K. Improved outcome following preoperative radiochemotherapy: 40.5 Gy accelerated hyperfractionation and 5-fluorouracil suppositories for patients with carcinoma of the lower rectum. Surg Today. 29:992–998. 1999.

20. Eypasch E, Wood-Dauphinee S, Williams JI, Ure B, Neugebauer E, Troidl H. The Gastrointestinal Quality of Life Index. A clinical index for measuring patient status in gastroenterologic surgery. Chirurg. 64:264–274. 1993.
Go to : 
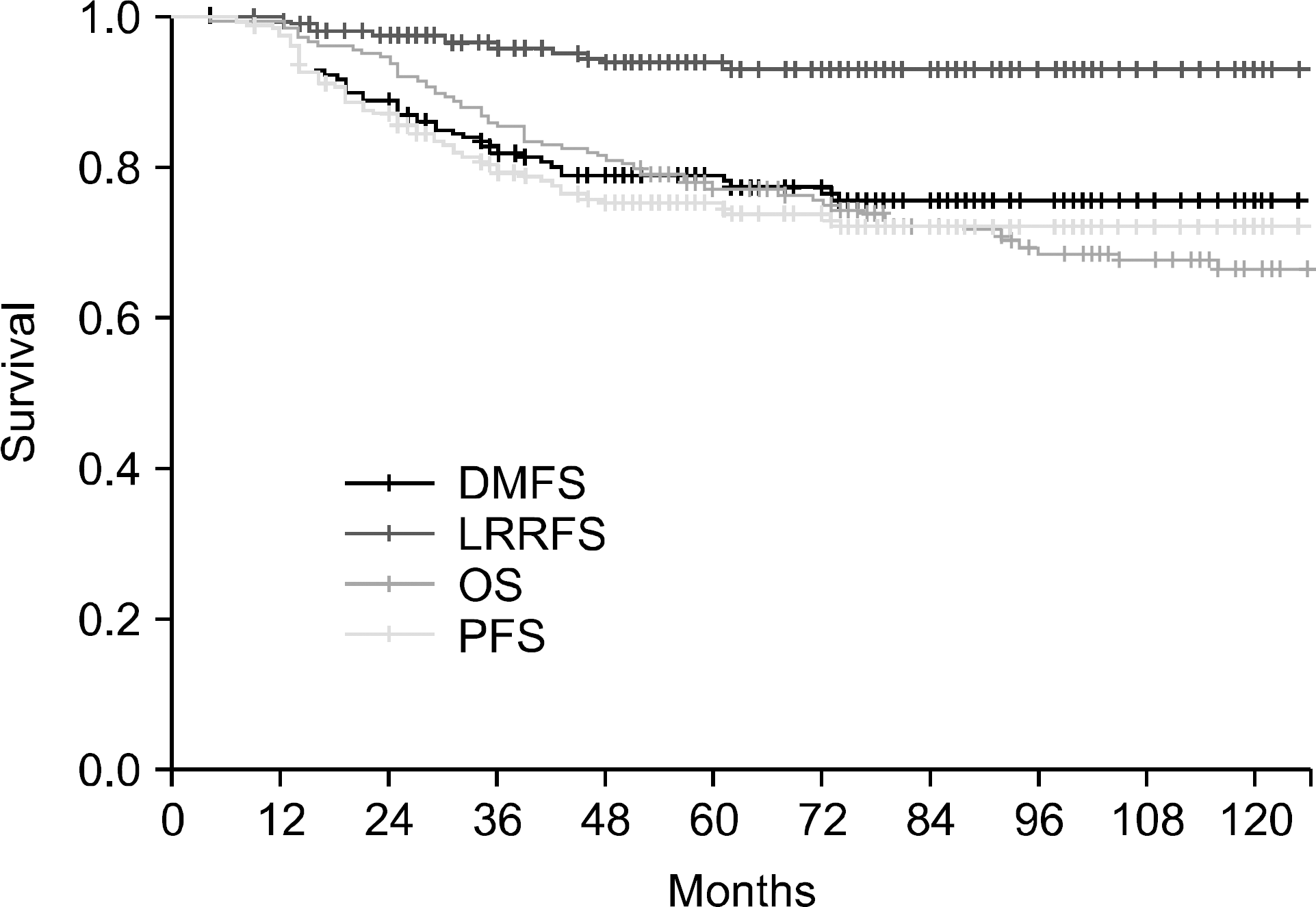 | Fig. 1.Overall, disease-free, locoregional relapse-free, and distant metastasis-free survival rates of all patients. |
Table 1.
Patient and tumor characteristics.
Table 2.
Complications after surgery.
Table 3.
Ileostomy or colostomy status in patients wh received sphincter preservation surgery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download