Abstract
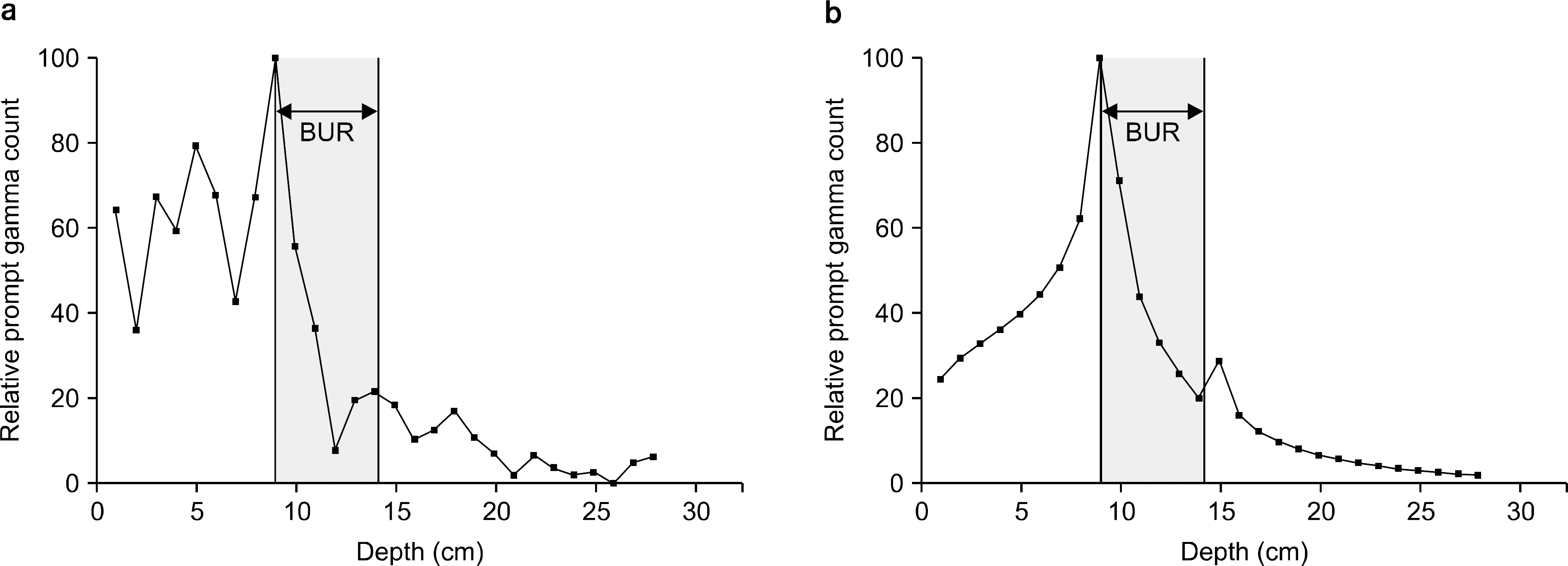
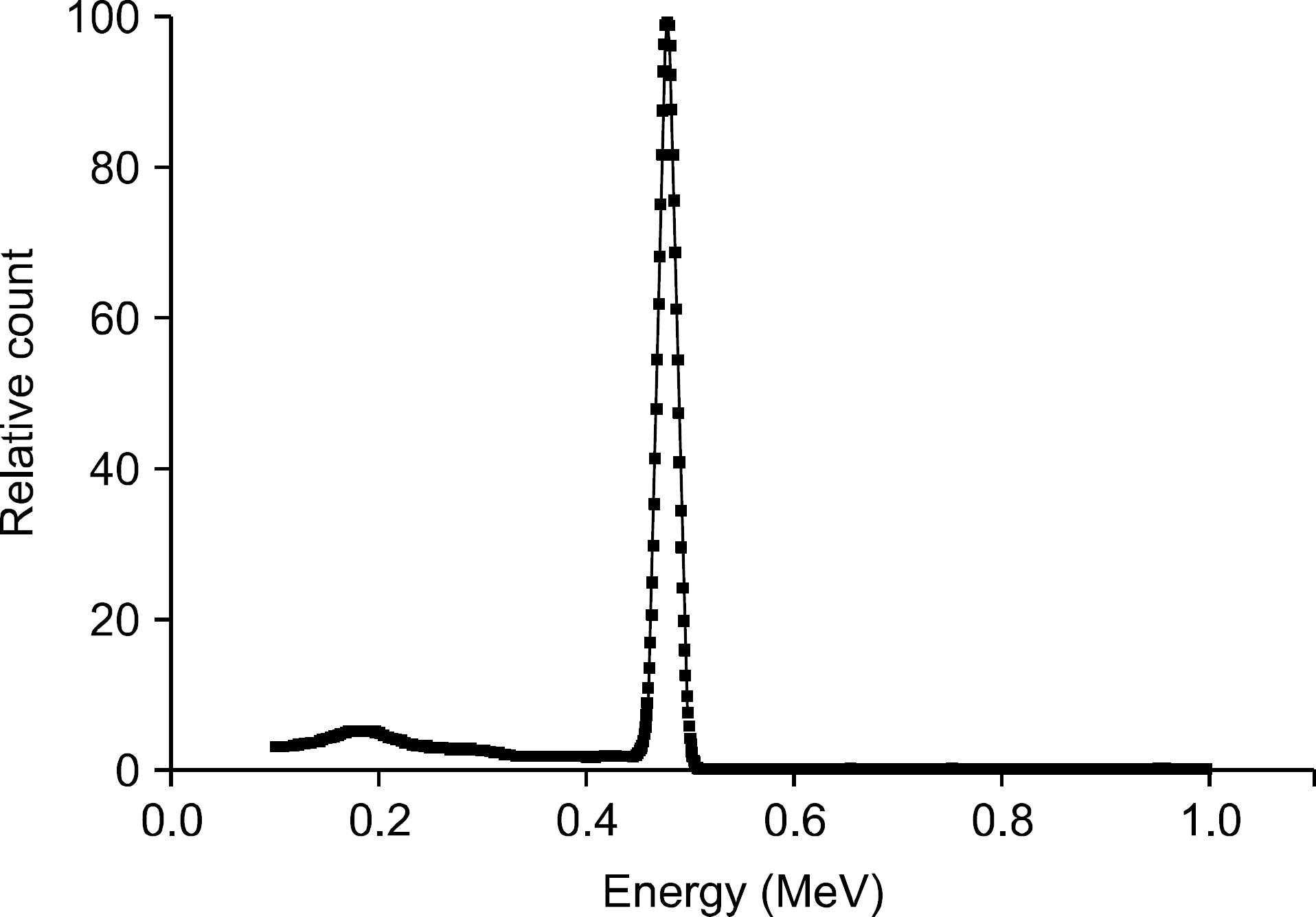
The purpose of this study was to confirm the feasibility of imaging of therapy region from the boron neutron capture therapy (BNCT) using the measurement of the prompt gamma ray depending on the neutron flux. Through the Monte Carlo simulation, we performed the verification of physical phenomena from the BNCT; (1) the effects of neutron according to the existence of boron uptake region (BUR), (2) the internal and external measurement of prompt gamma ray dose, (3) the energy spectrum by the prompt gamma ray. All simulation results were deducted using the Monte Carlo n-particle extended (MCNPX, Ver.2.6.0, Los Alamos National Laboratory, Los Alamos, NM, USA) simulation tool. The virtual water phantom, thermal neutron source, and BURs were simulated using the MCNPX. The energy of the thermal neutron source was defined as below 1 eV with 2,000,000 n/sec flux. The prompt gamma ray was measured with the direction of beam path in the water phantom. The detector material was defined as the lutetium-yttrium oxyorthosilicate (Lu0,6Y1,4Si0,5:Ce; LYSO) scintillator with lead shielding for the collimation. The BUR's height was 5 cm with the 28 frames (bin: 0.18 cm) for the dose calculation. The neutron flux was decreased dramatically at the shallow region of BUR. In addition, the dose of prompt gamma ray was confirmed at the 9 cm depth from water surface, which is the start point of the BUR. In the energy spectrum, the prompt gamma ray peak of the 478 keV was appeared clearly with full width at half maximum (FWHM) of the 41 keV (energy resolution: 8.5%). In conclusion, the therapy region can be monitored by the gamma camera and single photon emission computed tomography (SPECT) using the measurement of the prompt gamma ray during the BNCT.
References
1. Ono K, Kinashi Y, Masunaga S, Suzuki M, Takagaki M. Electroporation increases the effect of borocaptate (10B-BSH) in neutron capture therapy. Int. J. Radiat. Oncol. Biol. Phys. 42:823–826. 1998.
2. Barth RF, Soloway AH, Goodman JH, Gahbauer RA, Gupta N, Blue TE, et al. Boron neutron capture therapy of brain tumors: an emerging therapeutic modality. Neurosurgery. 44:433–503. 1999.

3. Barth RF, Grecula JC, Yang W, Rotaru JH, Nawrocky M, Gupta N, et al. Combination of boron neutron capture therapy and external beam radiotherapy for brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 58:267–277. 2004.

4. Sherlock Huang LC, Hsieh WY, Chen JY, et al. Drug delivery system design and development for boron neutron capture therapy on cancer treatment. Appl. Radiat. Isot. 88:89–93. 2014.

5. Yokoyama K, Miyatake S, Kajimoto Y, et al. Pharmacokinetic study of BSH and BPA in simultaneous use for BNCT. J. Neurooncol. 78:227–232. 2006.

6. Munck af Rosenschöld PM, Verbakel WFAR, Ceberg CP, et al. Toward clinical application of prompt gamma spectroscopy for in vivo monitoring of boron uptake in boron neutron capture therapy. Med. Phys. 28:787–795. 2001.
7. Kinashi Y, Masunaga S, Nagata K, et al. A bystander effect observed in boron neutron capture therapy: a study of the induction of mutations in the HPRT locus. Int. J. Radiat. Oncol. Biol. Phys. 68:508–514. 2007.

8. Kankaanranta L, Seppala T, Koivunoro H, et al. Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: final analysis of a phase I/II trial. Int. J. Radiat. Oncol. Biol. Phys. 82:e67–e75. 2012.

9. Culbertson C. N., Green, S., Mason, A. J., Picton, D., Baugh, G., Hugtenburg, R. P., Nelson, J. M.: In-phantom characterisation studies at the Birmingham Accelerator-Generated epIthermal Neutron Source (BAGINS) BNCT facility. Appl. Radiat. Isot. 61:733–738. 2004.
10. Moss R. L., Stecher-Rasmussen, F., Ravensberg, K., Constantine, G., Watkins, P.: Design, construction and installation of an epithermal neutron beam for BNCT at the highflux reactor Petten. Progress in Neutron Capture Therapy for Cancer. Springer US;p. 63–66. 1992.
11. Bartoli , A. , Belcari , N. , Del Guerra, A. , and Fabbri, S.: Simultaneous PET/SPECT imaging with the small animal scanner YAP-(S) PET. IEEE Nuclear Science Symposium Conference Record, 2007 NSS. 5:3408–3413. 2007.
12. Yao R, Deng X, Beaudoin J-F, Ma T, Cadorette J, Cao Z, et al. Initial Evaluation of LabPET/SPECT Dual Modality Animal Imaging System. IEEE Transactions on Nuclear Science. 60:76–81. 2013.

13. Giuliano D. R.: Neutron Flux Measurements and Calculations in the Gamma Irradiation Facility Using MCNPX. Doctoral dissertation, University of Cincinnati. (. 2010.
14. Pidol L, Kahn-Harari A, Viana B, Virey E, Ferrand B, Dorenbos P, et al. High efficiency of lutetium silicate scintillators, Ce-doped LPS and LYSO crystals. IEEE Nuclear Science Symposium Conference Record. 2:886–890. 2003.

15. Chewpraditkul , W. , Swiderski , L. , Moszynski , M. , Szczesniak , T. , Syntfeld-Kazuch , A. , Wanarak , C. , & Limsuwan P. Scintillation properties of LuAG: Ce, YAG: Ce and LYSO: Ce crystals for gamma-ray detection. Nuclear Science, IEEE Transactions on. 56:3800–3805. 2009.
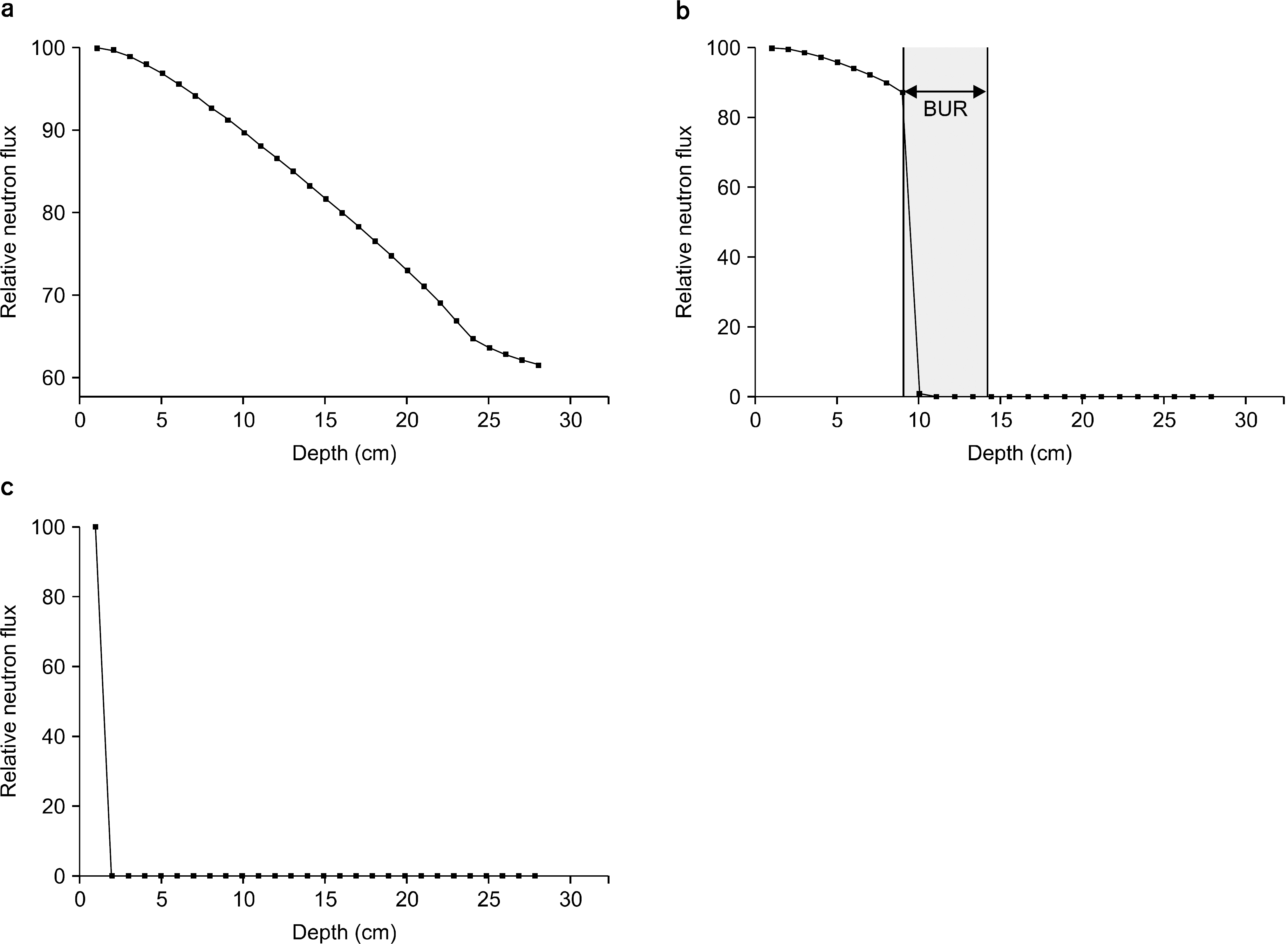
Fig. 1.
Change of the neutron flux depending on the depth of the phantom. (a) The neutron flux according to the depth from the water phantom without boron uptake region (BUR), (b) The neutron flux according to the depth from the water phantom with boron uptake region (BUR), (c) the change of the neutron flux from the BUR.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download