Abstract
Hyperthermia is effective treatment modality when it combine with the radiotherapy treatment. It is important to verify the temperature distribution of (patient's) body for the safety and effective treatment during raising the temperature. In this study, we raised the temperature in agar phantom using radio frequency (RF) Hyperthermia and protocol that manufacture recommend. Temperature distribution measured 5 section of 5 cm thickness with agar phantom. When the temperature was raised according to the increase energy. Temperature distribution was elevated at similar domain regardless of energy. The temperature tend to be increased at up side then bottom side and also increase when A large electrode was used than small one.
Go to : 
REFERENCES
1. de Bruijne M, Van der Zee J, Ameziane A, Van Rhoon GC. Schlag PM: Quality control of superficial hyperthermia by treatment evaluation: Int J Hyperthermia. 27(3):199–213. 2011.
2. Overgaard J, Gonzalez Gonzalez D, Hulshof MCCM, et al. Trial of hyperthermia as adjuvant to radiotherapy for recurrentor metastatic malignant melanoma. Lancet. 345:540–543. 1995.
3. Vernon CC, Hand JW, Field SB, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer - results from five randomised controlled trials. Int J Radiation Oncology Biol Phys. 35:731–744. 1996.
4. Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 23:3079–3085. 2005.

5. 추성실, 김귀언, 노준규. 암 치료를 위한 RF 온열장치의 개발과 가온 특성. 대한암학회지. 18(2):183–193. 1986.
6. Wust P, Hildebrandt B, Sreenivasa G, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 3(8):487–97. 2002.

7. Rietveld PJM, Van Putten WLJ, Van der Zee J, Van Rhoon GC. Comparison of the clinical effectiveness of the 433 Mhz lucite cone applicator with that of a conventional waveguide applicator in applications of superficial hyperthermia. Int J Radiat Oncol Biol Phys. 43:681–687. 1999.

8. Lee WM, Gelvich EA, Van der Baan P, Mazokhin VN, Van Rhoon GC. Assessment of the performance characteristics of a prototype 12-element capacitive contact flexible mi crostrip applicator (CFMA-12) for superficial hyperthermia. Int J Hyperthermia. 20:607–624. 2004.
9. Diederich CJ, Stauffer PR. Pre-clinical evaluation of a microwave planar array applicator for superficial hyperthermia. Int J Hyperthermia. 9:227–246. 1993.

10. Lee ER, Wilsey TR, Tarczy-Hornoch P, et al. Body conformable915MHz microstrip array applicators for large surface areahyperthermia. IEEE Trans Biomed Eng. 39:470–483. 1992.
11. Samulski TV, Fessenden P, Lee ER, Kapp DS, Tanabe E, McEuen A. Spiral microstrip hyperthermia applicators: Technical design and clinical performance. Int J Radiat Oncol Biol Phys. 18:233–42. 1990.

12. Rossetto F, Stauffer PR. Theoretical characterization of dual concentric conductor microwave applicators for hyperthermia at 433 MHz. Int J Hyperthermia. 17:258–270. 2001.
13. Jacobsen S, Stauffer PR, Neuman DG. Dual-mode antenna design for microwave heating and noninvasive thermometry of superficial tissue disease. IEEE Trans Biomed Eng. 47:1500–1509. 2000.

Go to : 
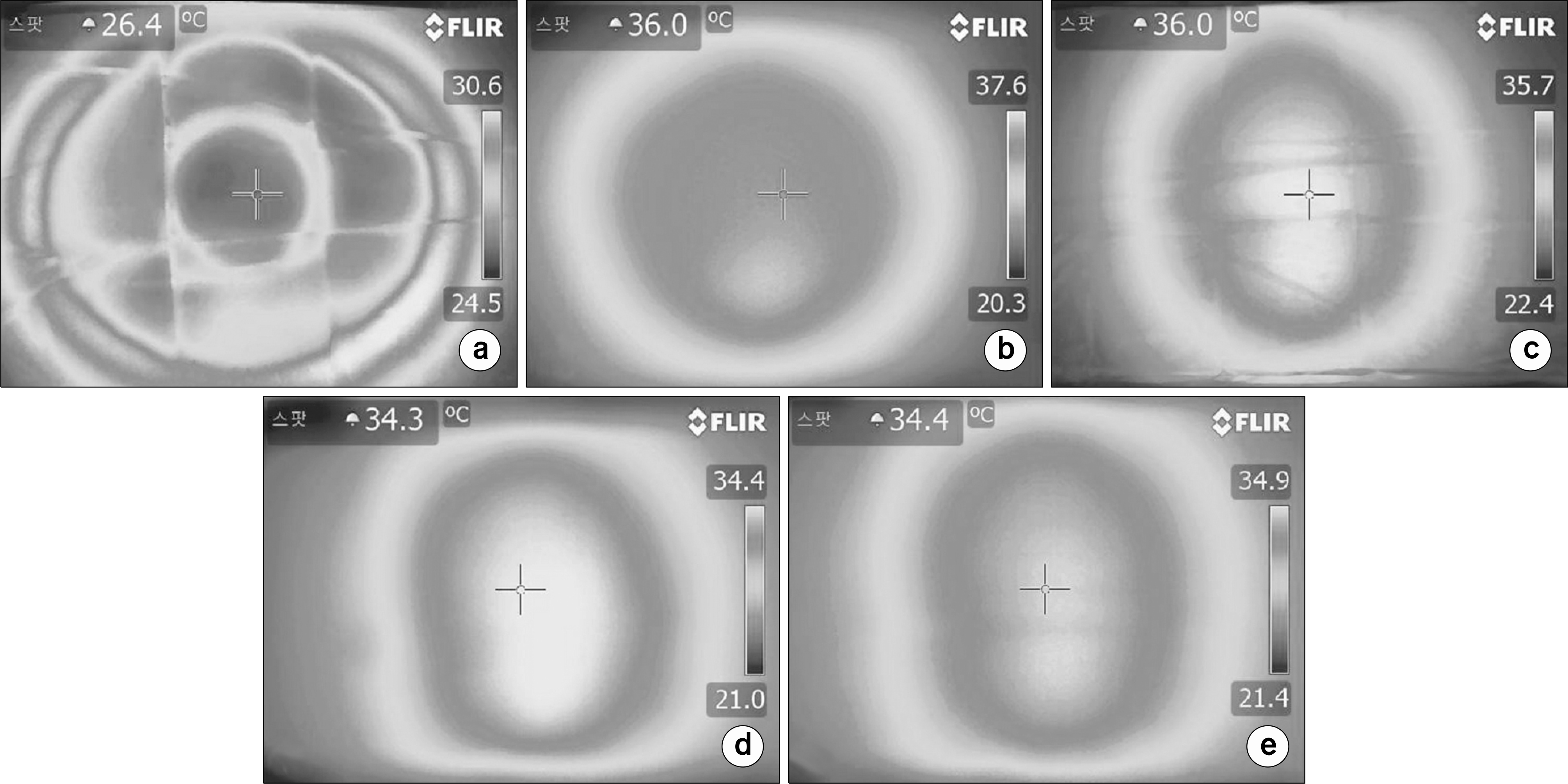 | Fig. 2.Thermal distribution of 25∼25 cm electrode (liver protocol session 5), (a) surface, (b) depth 3 cm, (c) depth 6 cm, (d) depth 9 cm, (e) depth 12 cm. |
 | Fig. 3.Temperature Profiles (liver protocol session 5), (a) electrode 25∼25 cm, (b) electrode 15∼15 cm. |
 | Fig. 4.Thermal distribution of 15∼15 cm electrode (liver protocol session 5), (a) surface, (b) depth 3 cm, (c) depth 6 cm, (d) depth 9 cm, (e) depth 12 cm. |
 | Fig. 5.Thermal distribution of 15∼25 cm electrode (liver protocol session 5), (a) surface, (b) depth 3 cm, (c) depth 6 cm, (d) depth 9 cm, (e) depth 12 cm. |
 | Fig. 6.Thermal distribution of 25-15 cm electrode (liver protocol session5), (a) surface, (b) depth 3 cm, (c) depth 6 cm, (d) depth 9 cm, (e) depth 12 cm. |
Table 1.
Measurement conditions of thermal distribution.
| Diameter of electrodes (cm) | Duration (min) | Power (watt) | |
|---|---|---|---|
| Upper-lower | |||
| Liver protocol session 1 | 25-25 | 10 | 35 |
| 10 | 55 | ||
| 10 | 75 | ||
| 10 | 85 | ||
| 10 | 95 | ||
| Liver protocol session 5 | 25-25 | 10 | 60 |
| 10 | 85 | ||
| 10 | 110 | ||
| 10 | 135 | ||
| 10 | 140 |
Table 2.
Measurement conditions of thermal distribution.
| Diameter of electrodes (cm) | Duration (min) | Power (watt) | |
|---|---|---|---|
| Head & Neck protocol session 5 | Upper-lower | 10 | 60 |
| 10 | 80 | ||
| 25-15 | 10 | 95 | |
| 15-15 | 10 | 110 | |
| 15-25 | 10 | 120 |
Table 3.
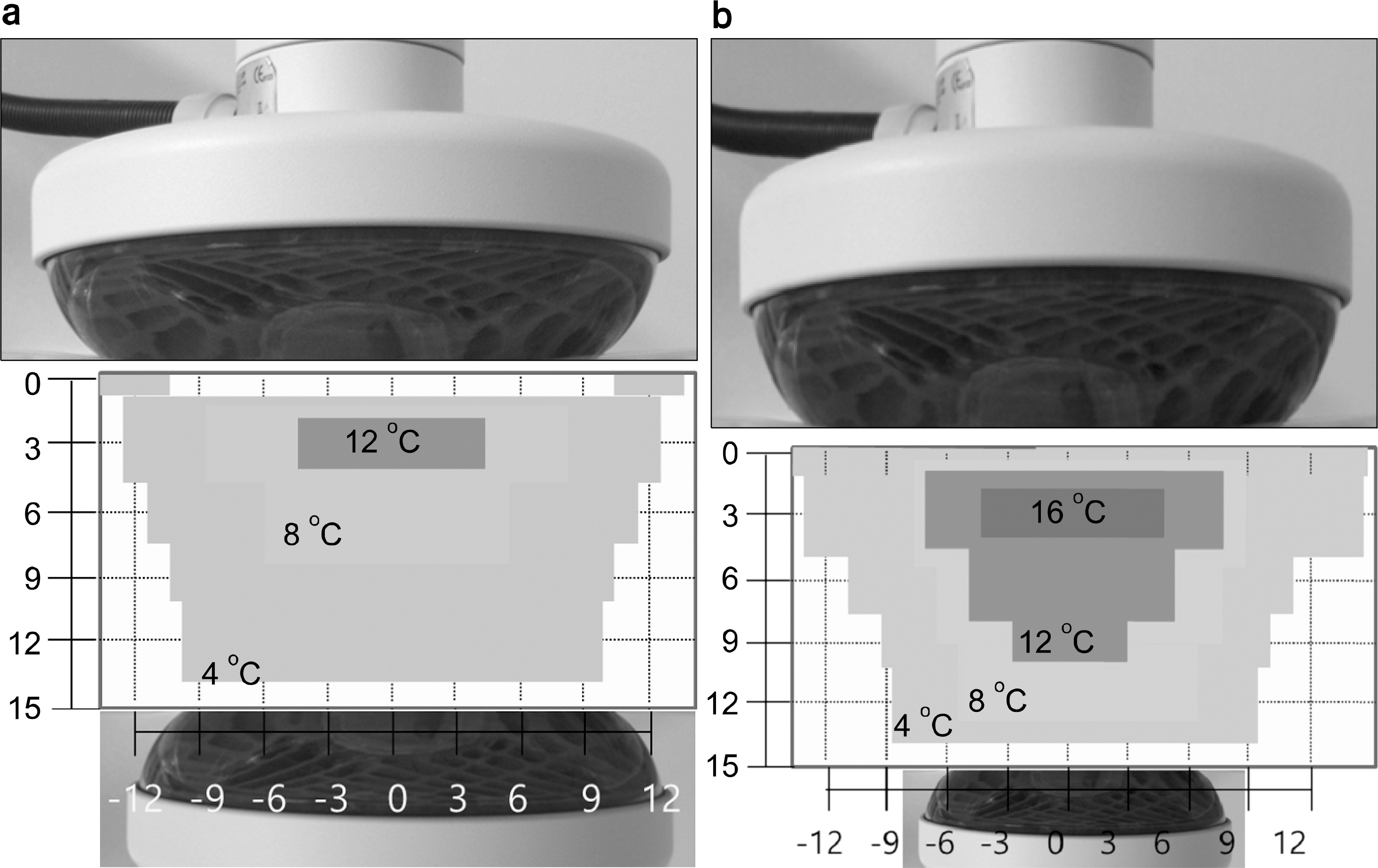
Thermal distribution at center of 25∼25 cm electrode, duration 10 min (a) Liver protocol session1 (total energy 206 kJ), (b) Liver protocol session5 (total energy 315 kJ).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download