Abstract
The surgical resection was occurred mainly in liver metastasis before the development of radiation therapy techniques. Recently, Radiation therapy is increased gradually due to the development of radiation dose delivery techniques. 18F-FDG PET image showed better sensitivity and specificity in liver metastasis detection. This image modality is important in the radiation treatment with planning CT for tumor delineation. In this study, we applied automatic image segmentation methods on PET image of liver metastasis and examined the impact of image factors on these methods. We selected the patients who were received the radiation therapy and 18F-FDG PET/CT in Korea Cancer Center Hospital from 2009 to 2012. Then, three kinds of image segmentation methods had been applied; The relative threshold method, the Gradient method and the region growing method. Based on these results, we performed statistical analysis in two directions. 1. comparison of GTV and image segmentation results. 2. performance of regression analysis for relation between image factor affecting image segmentation techniques. The mean volume of GTV was 60.9±65.9 cc and the GTV40% was 22.43±35.27 cc, and the GTV50% was 10.11±17.92 cc, the GTVRG was 32.89±36.84 cc, the GTVGD was 30.34±35.77 cc, respectively. The most similar segmentation method with the GTV result was the region growing method. For the quantitative analysis of the image factors which influenced on the region growing method, we used the standardized coefficient β, factors affecting the region growing method show GTV, TumorSUVMAX/MIN, SUVmax, TBR in order. The result of the region growing (automatic segmentation) method showed the most similar result with the CT based GTV and the region growing method was affected by image factors. If we define the tumor volume by the auto image segmentation method which reflect the PET image parameters, more accurate and consistent tumor contouring can be done. And we can irradiate the optimized radiation dose to the cancer, ultimately.
Go to : 
REFERENCES
1. H⊘yer M, Swaminath A, Bydder S, et al. Radiotherapy for liver metastases: A review of evidence. Int J Radiat Oncol Biol Phys. 82(3):1047–1057. 2012.
2. Lock MI, H⊘yer M, Bydder SA, et al. An international survey on liver metastases radiotherapy. Acta Oncologica. 51:568–574. 2012.

3. Liu LX, Zhang WH, Jiang HC. Current treatment for liver metastases from colorectal cancer. World J Gastroenterol. 9(2):193–200. 2003.

4. Gasent Blesa JM, Dawson LA. Options for radiotherapy in the treatment of liver metastases. Clin Transl Oncol. 10:638–645. 2008.

5. Parlak C, Topkan E, Sonmez S, Onal C, Reyhan M. CTversus coregistered FDG-PET/CT-based radiation therapy plans for conformal radiotherapy ib colorectal liver metastases: a dosimetric comparison. Jpn J Radiol. 30(8):628–634. 2012.
6. Zaidi H, Vees H, Wissmeyer M. Molecular PET/CT imaging- guided radiation therapy treatment planning. Acad Radiol. 16(9):L1108–1133. 2009.
7. Kao CH, Hsieh TC, Yu CY, et al. 18F-FDG PET/CT-based gross tumor volume definition for radiotherapy in head and neck cancer: a correlation study between suitable uptake value threshold and tumor parameters. Radiat Oncol. 76(5):2010.

8. Wanet M, Lee JA, Weynard B, et al. Gradient-based delineation of the primary GTV on FDG-PET in non-small cell lung cancer: a comparison with threshold-based approaches CT and surgical specimens. Radiother Oncol. 98(1):117–125. 2011.

9. Chua SC, Groves AM, Kayani I, et al. The impact of 18FFDG PET/CT in patients with liver metastases. Eur J Nucl Med Mol Imaging. 34:1906–1914. 2007.

10. Bipat S, van Leeuwen MS, Comans EF, et al. Colorectal liver metastases: CT, MR imaging and PET for diagnosismeta- analysis. Radiology. 237(1):123–131. 2005.
11. Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): A metaanalysis. Radiology. 224(3):748–756. 2002.

12. Grégoire V, Haustermans K, Geets X, Roels S, Lonneux M. PET-based treatment planning in radiotherapy: A new standard? J Nucl Med. 48(1):68S–77S. 2007.
13. Lee JA. Segmentation of positron emission tomography image: some recommendations for target delineation in radiation oncology. Radiother Oncol. 96(3):302–307. 2010.
14. Zaidi H, Naqa IE. PET-guided delineation of radiation therapy treatment volumes: a survey of image segmentation techniques. Eur J Nucl Med Imaging. 37(11):2165–2187. 2010.

15. Hatt M, Cheze-le Rest C, van Baardwijk A, Lambin P, Pradier O, Visvikis D. Impact of tumor size and tracer uptake heterogeneity in (18)F-FDG PET and CT non-small cell lung cancer tumor delineation. J Nucl Med. 52(11):1690–1697. 2011.

16. Vees H, Senthamizhchelvan S, Miralbell R, Wever DC, Ratib O, Zaidi H. Assessment of various strategies for 18F-FET PET-guided delineation of target volumes in highgrade glioma patients. Eur J Nucl Med Mol Imaging. 36(2):182–193. 2009.

17. Hong R, Halama J, Bova D, Sethi A, Emami B. Correlation of PET standard uptake value and CT window-level thresholds for target delineation in CT-based radiation treatment planning. Int J Radiat Oncol Biol Phys. 67(3):720–726. 2007.

18. Nestle U, Kermp S, Schaefer-Schuler A, et al. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-small cell lung cancer. J Nucl Med. 46(8):1342–1348. 2005.
19. van Baardwijk A, Baumert BG, Bosmans G, et al. The current status of FDG-PET in tumour volume definition in radiotherapy treatment planning. Cancer Treat Rev. 32:245–260. 2006.

20. Geets X, Lee JA, Lonneux M, Grégoire V. A gradientbased method for segmenting FDG-PET images: methodology and validation. Eur J Nucl Med Mol Imageing. 34(9):1427–1438. 2007.

21. Paulino AC, Koshy M, Howell R, Schuster D, Davis LW. Comparison of CT- and FDG-PET-defined gross tumor volume in intensity modulated radiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 61(5):1385–1392. 2005.
22. Bassi MC, Turri L, Sacchetti G, et al. FDG-PET/CT imaging for staging and target volume delineation in preoperative conformal radiotherapy of rectal cancer. Int J Radiat Oncol Biol Phys. 70(5):1423–1426. 2008.

23. Day E, Betler J, Parda D, et al. A region growing method for tumor volume segmentation on PET images for rectal and anal cancer patients. Med Phys. 36(10):4349–4358. 2009.

24. Graves EE, Quon A, Loo BW Jr. RT_Image: an opensource tool for investigating PET in radiation oncology. Technol Cancer Res Treat. 6(2):111–121. 2007.

25. Gonzalez RC, Woods RE. 디지털 영상처리 3판, 유현중 등: 피어슨에듀케이션코리아. 서울(2009), pp. 839–900.
27. Ariff B, Lloyd CR, Khan S, et al. Imaging of liver cancer. World J Gastroenterol. 15(11):1289–1300. 2009.

28. Biehl KJ, Kong FM, Dehdashti F, et al. 18F-FDG PET definition of gross tumor volume for radiotherapy of non-small cell lung cancer: is a single standardized uptake value threshold approach appropriate? J Nucl Med. 47(11):1808–1812. 2006.
29. Basu S, Kwee TC, Gatenby R, Saboury B, Torigian DA, Alavi A. Evolving role of molecular imaging with PET in detecting and characterizing heterogeneity of cancer tissue at the primary and metastatic sites, a plausible explanation for failed attempts to cure malignant disorders. Eur J Nucl Med Mol Imaging. 38:987–991. 2011.

30. Ling CC, Humm J, Larson S, et al. Towards multiidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Bio Phys. 47(3):551–560. 2000.
31. Daisne JF, Sibomana M, Bol A, Doumont T, Lonneux M, Grégoire V. Tri-dimensional autometic segmentation of PET volumes based on measured source-to-background ratios: influence of reconstruction algorithms. Radiother Oncol. 69(3):247–250. 2003.
32. Brambilla M, Matheoud R, Secco C, Loi G, Kerengill M, Inglese E. Threshold segmentation for PET target volume delineation in radiation treatment planning: the role of targetto- background ratio and target size. Med Phys. 35(4):1207–1213. 2008.
Go to : 
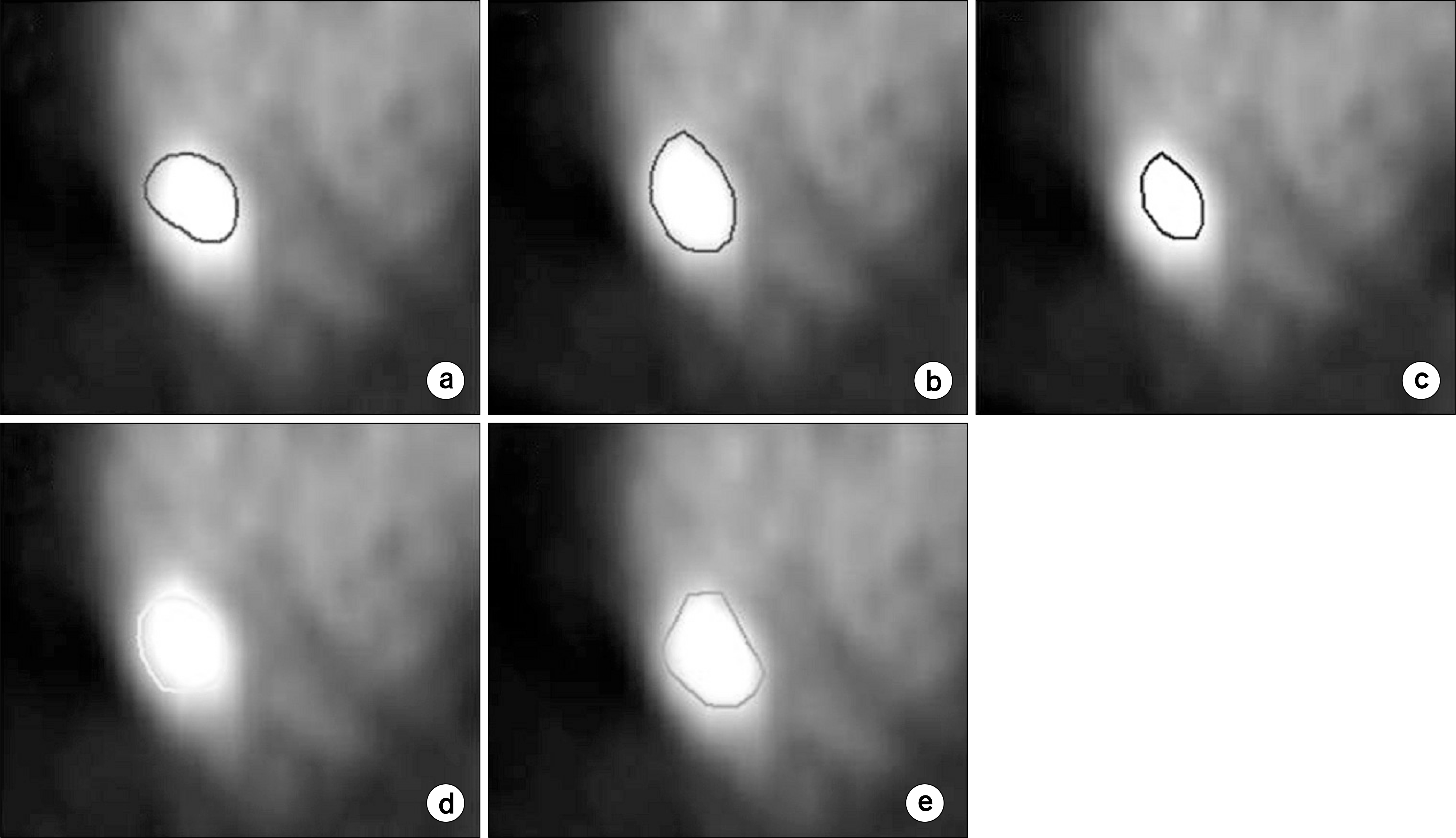 | Fig. 1.Applying automatic image segmentation in PET image. (a) CT based GTV, (b) relative threshold method (40%), (c) relative threshold method (50%), (d) gradient method, and (e) region growing method. |
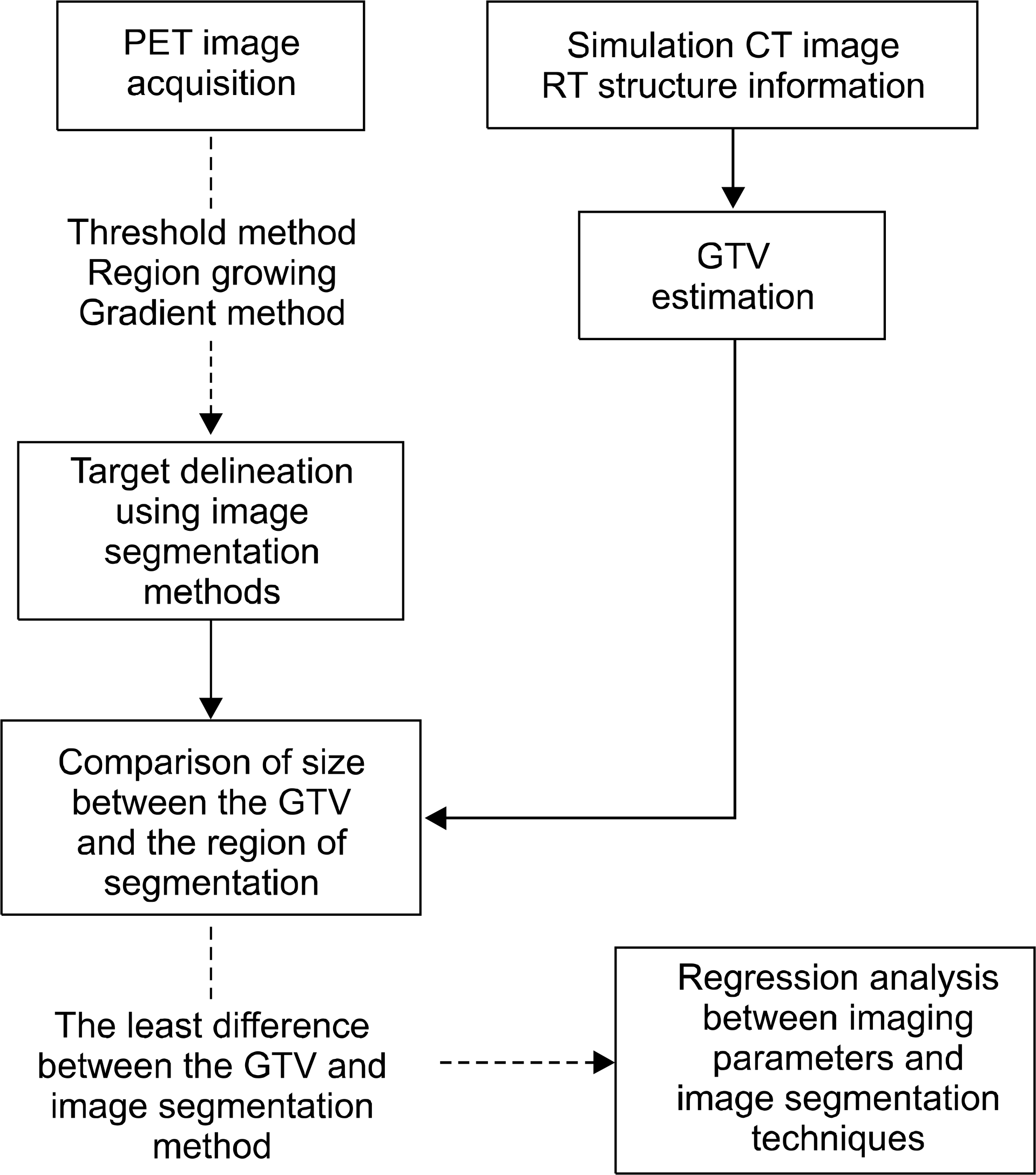 | Fig. 2.Flow chart for tumor delineation and statistical analysis using image segmentation methods. |
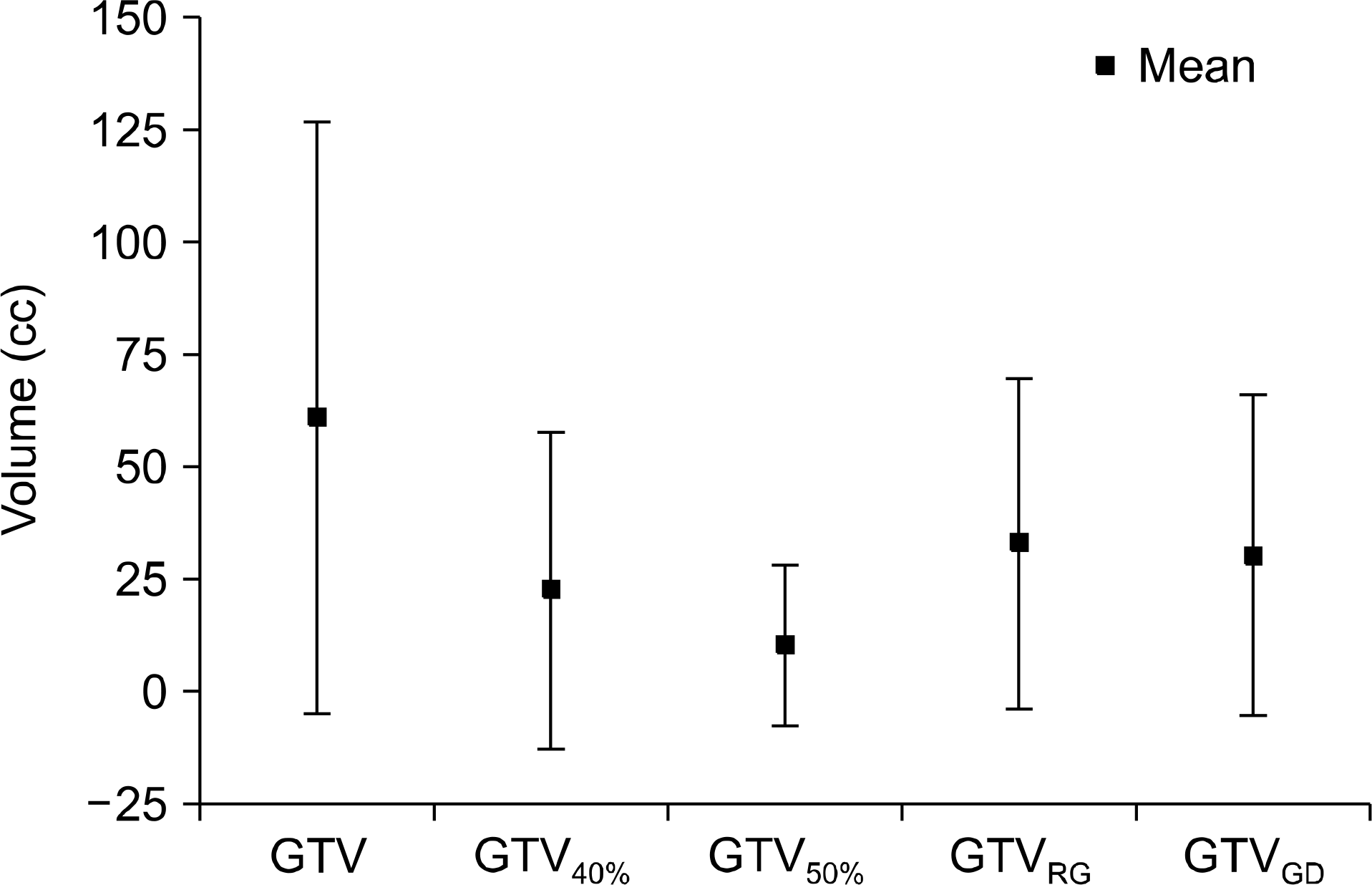 | Fig. 3.Comparison of mean tumor volumes. GTV range is 3.2 cc∼106.0 cc and mean volume is 60.9±65.9 cc, mean volume of GTV40%, GTV50%, GTVRG and GTVGD is 22.4±35.3 cc, 10.1±17.9 cc, 32.9±36.8 cc, and 30.3±35.8 cc respectively. |
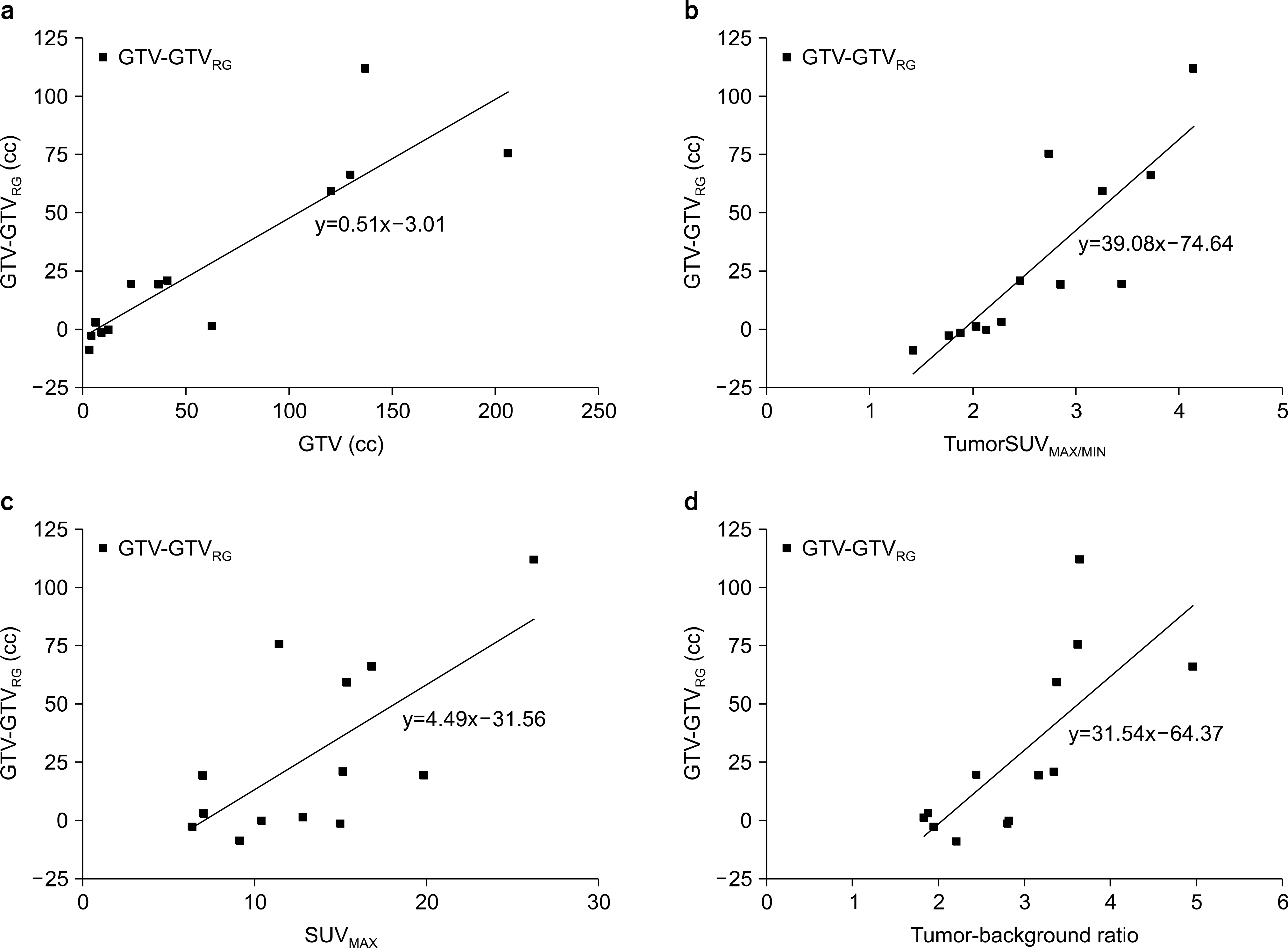 | Fig. 4.Correlation between GTV-GTVRG and image parameter. each graph means regression analysis results; (a) GTV, (b) TumorSUVMAX/MIN, (c) SUVMAX, (d) Tumor-Background Ratio. |
Table 1.
Patients's characteristics.
Table 2.
Results of automatic image segmentations. CT based GTV is reference volume, and volume of region growing is best fitness in GTV.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download