Abstract
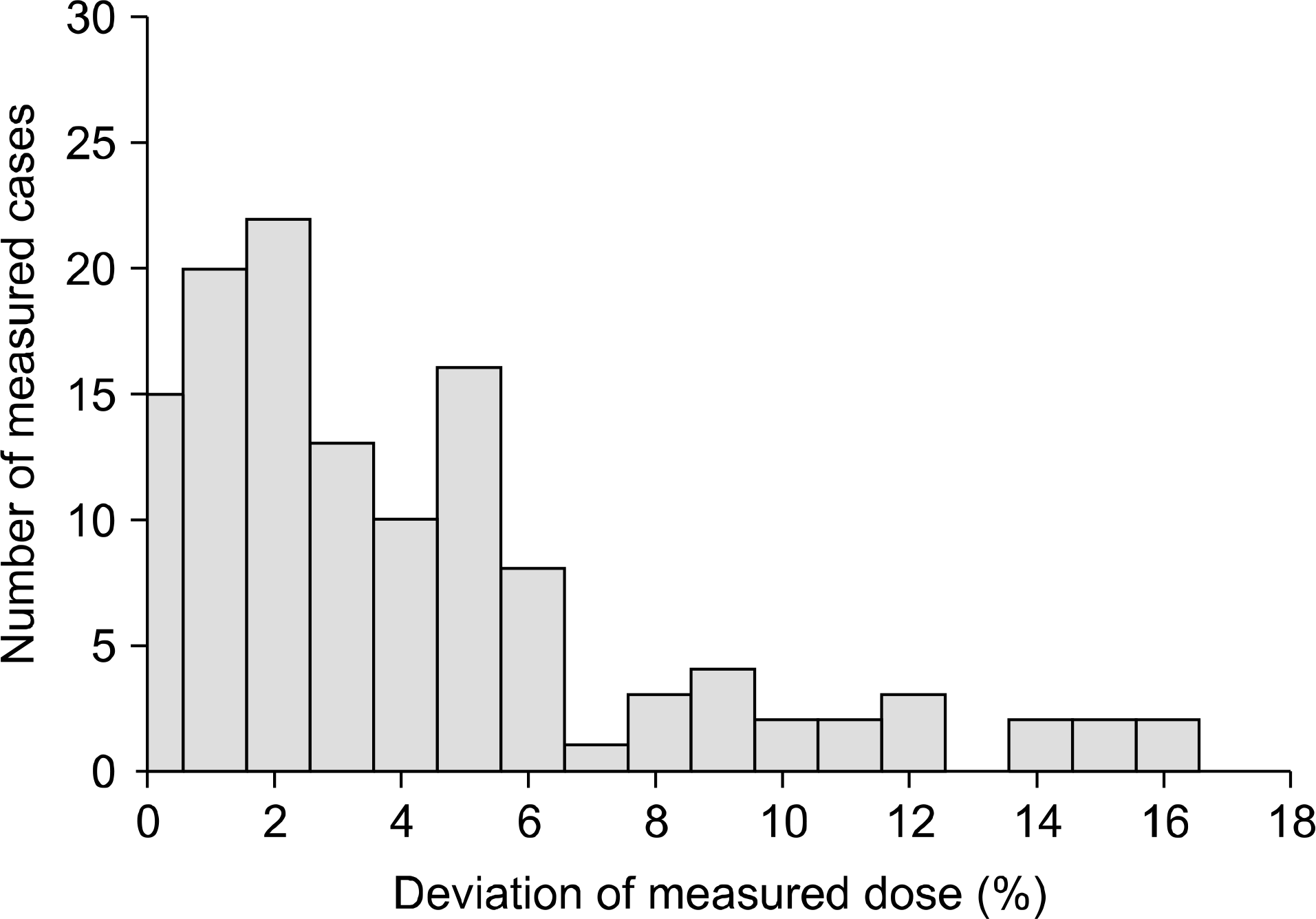
The experimental verification of treatment planning on the treatment spot is the ultimate method to assure quality of radiotherapy, so in-vivo skin dose measurement is the essential procedure to confirm treatment dose. In this study, glass rod dosimeter (GRD), which is a kind of photo-luminescent based dosimeters, was studied to produce a guideline to use GRDs in vivo dosimetry for quality assurance of radiotherapy. The pre-processing procedure is essential to use GRDs. This is a heating operation for stabilization. Two kinds of pre-processing methods are recommended by manufacturer: a heating method (70 degree, 30 minutes) and a waiting method (room temperature, 24 hours). We equally irradiated 1.0 Gy to 20 GRD elements, and then different preprocessing were performed to 10 GRDs each. In heating method, reading deviation of GRDs at same time were relatively high, but the deviation was very low as time went on. In waiting method, the deviation among GRDs was low, but the deviation was relatively high as time went on. The meaningful difference was found between mean reading values of two pre-processing methods. Both methods present mean dose deviation under 5%, but the relatively high effect by reading time was observed in waiting method. Finally, GRD is best to perform in-vivo dosimetry in the viewpoint of accuracy and efficiency, and the understanding of how pre-processing affect the accuracy is asked to perform most accurate in-vivo dosimetry. The further study is asked to acquire more stable accuracy in spite of different irradiation conditions for GRD usage.
Go to : 
REFERENCES
1. Mobit PN, Nahum AE, Mayles P. A MonteCarlo study of quality dependence factors of common TLD materials in photon and electron beams. Phys Med Biol. 43:2015–2032. 1998.
2. Kirby TH, Hanson WF, Johnston DA. Uncertainty analysis of absorbed dose calculations from thermoluminescence dosimeters. Med Phys. 19:1427–1433. 1992.

3. Rah JE, Kim SY, Cheong KH, et al. Feasibility study of radiophotoluminescent glass rod dosimeter postal dose intercomparison for high energy photon beam. Appl Radiat Isotopes. 67:324–328. 2009.

4. Rah JE, Suh WS, Shin DO, et al. Determination of output factors for the gamma knife using a radiophotoluminescent glass rod detector. Korean J Med Phys. 18:13–19. 2007.
5. Ko YE, Park SH, Choi BJ, et al. Comparison of skin dose measurement using glass rod dosimeter and diode for breast cancer patients. Korean J Med Phys. 19:9–13. 2008.
6. Raj V, John M, Brenden G, Michael W. In vivo prostate IMRT dosimetry with MOSFET detectors using brass buildup caps. J App Cli Med Phys. 7:22–32. 2006.
7. Shih H, Chien Y, Tien Y, et al. Clinical application of radiophotoluminescent glass dosimeter for dose verification of prostate HDR procedure. Med Phys. 35:5558–5564. 2008.
8. Arakia F, Moribe N, Shimonobou T, Yamashita Y. Dosimetric properties of radiophotoluminescent glass rod dosimeter in high-energy photon beams from a linear accelerator and cyber-knife. Med Phys. 31:1980–1986. 2004.
9. Mizunoa H, Kanaia T, Kusanob Y, et al. Feasibility study of glass dosimeter postal dosimetry audit of high-energy radiotherapy photon beams. Radiother Oncol. 86:258–263. 2007.
10. Technical Report. Explanation material of RPL glass dosimeter. Small Element System. Asahi Techno Glass Corporation, Tokyo, Japan. 2000.
Go to : 
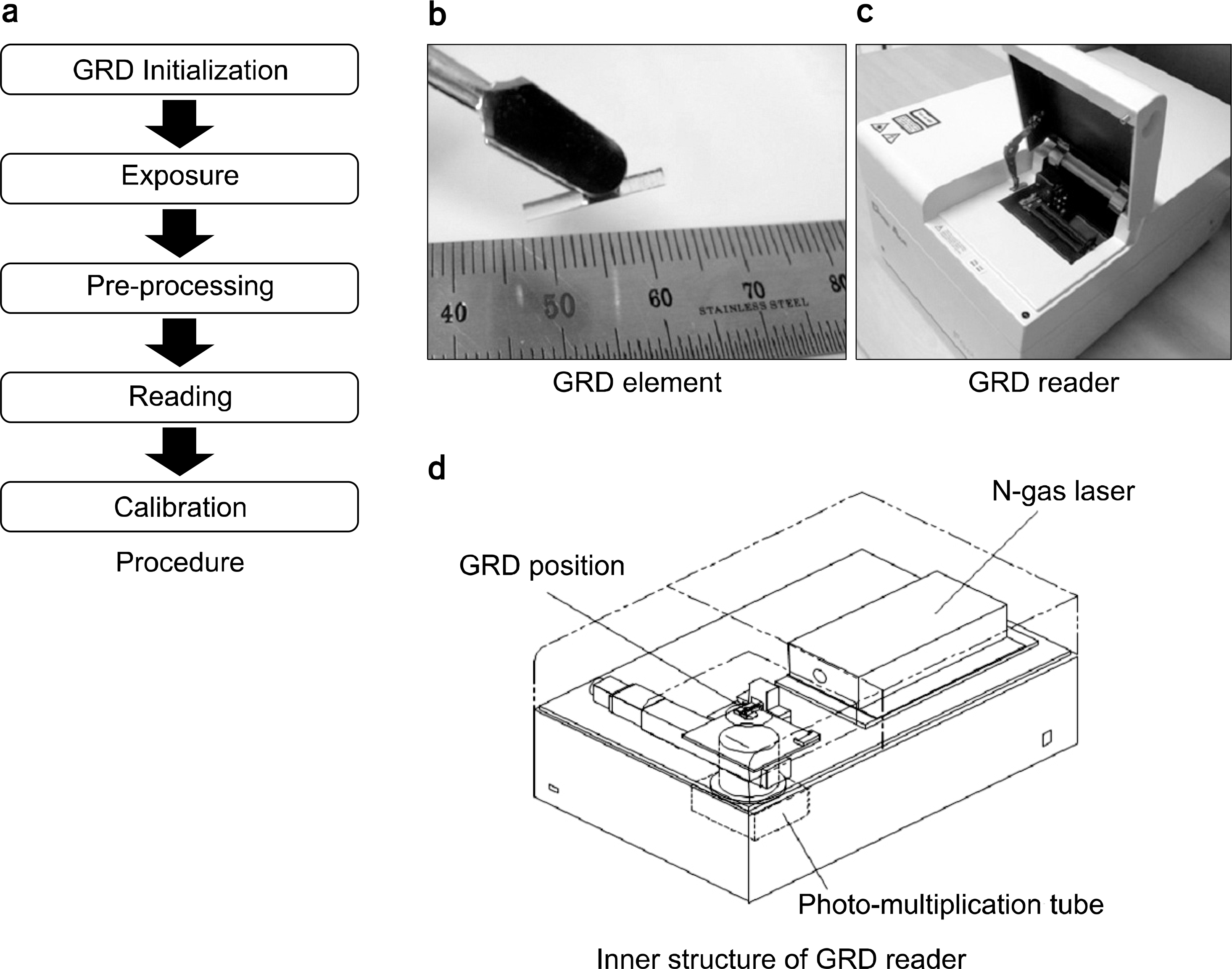 | Fig. 1.(a) The dose measurement procedure, (b) a GRD element, (c) a GRD reader, and (d) inner structure of the reader. |
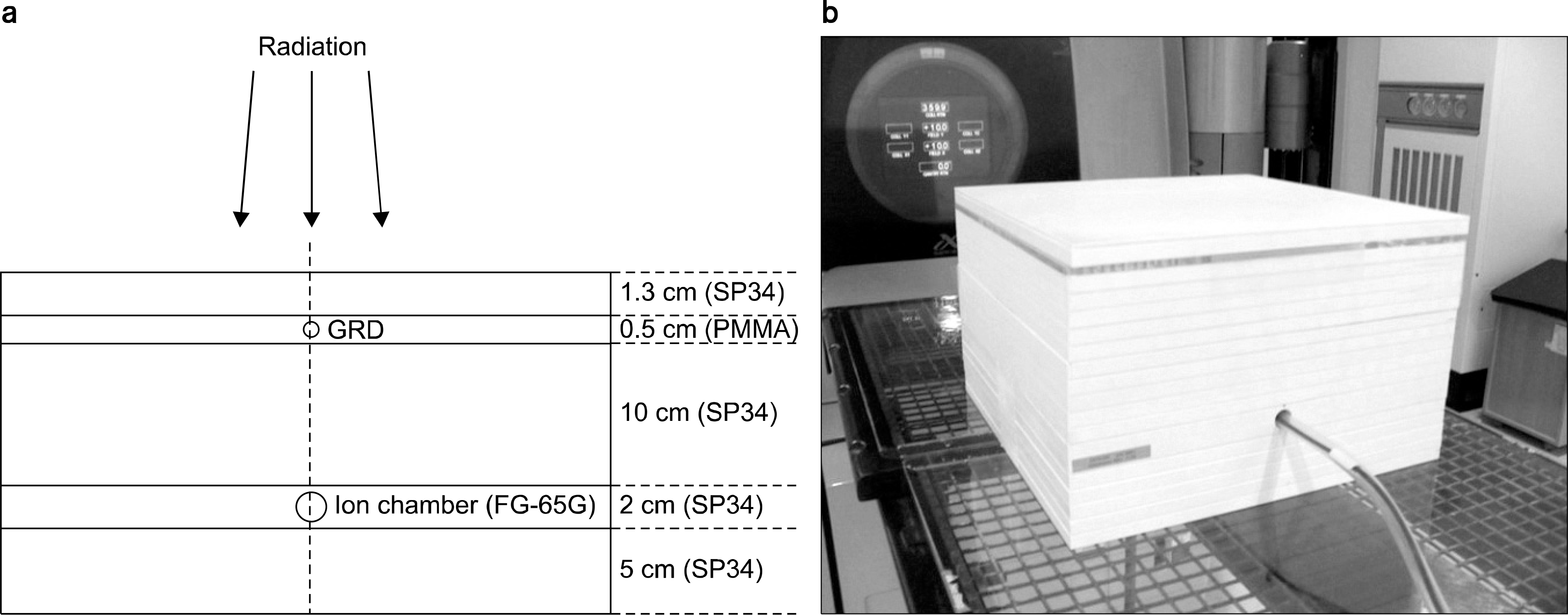 | Fig. 2.(a) Experimental setup of GRD elements and an ion chamber for radiation exposure and (b) its actual figure. |
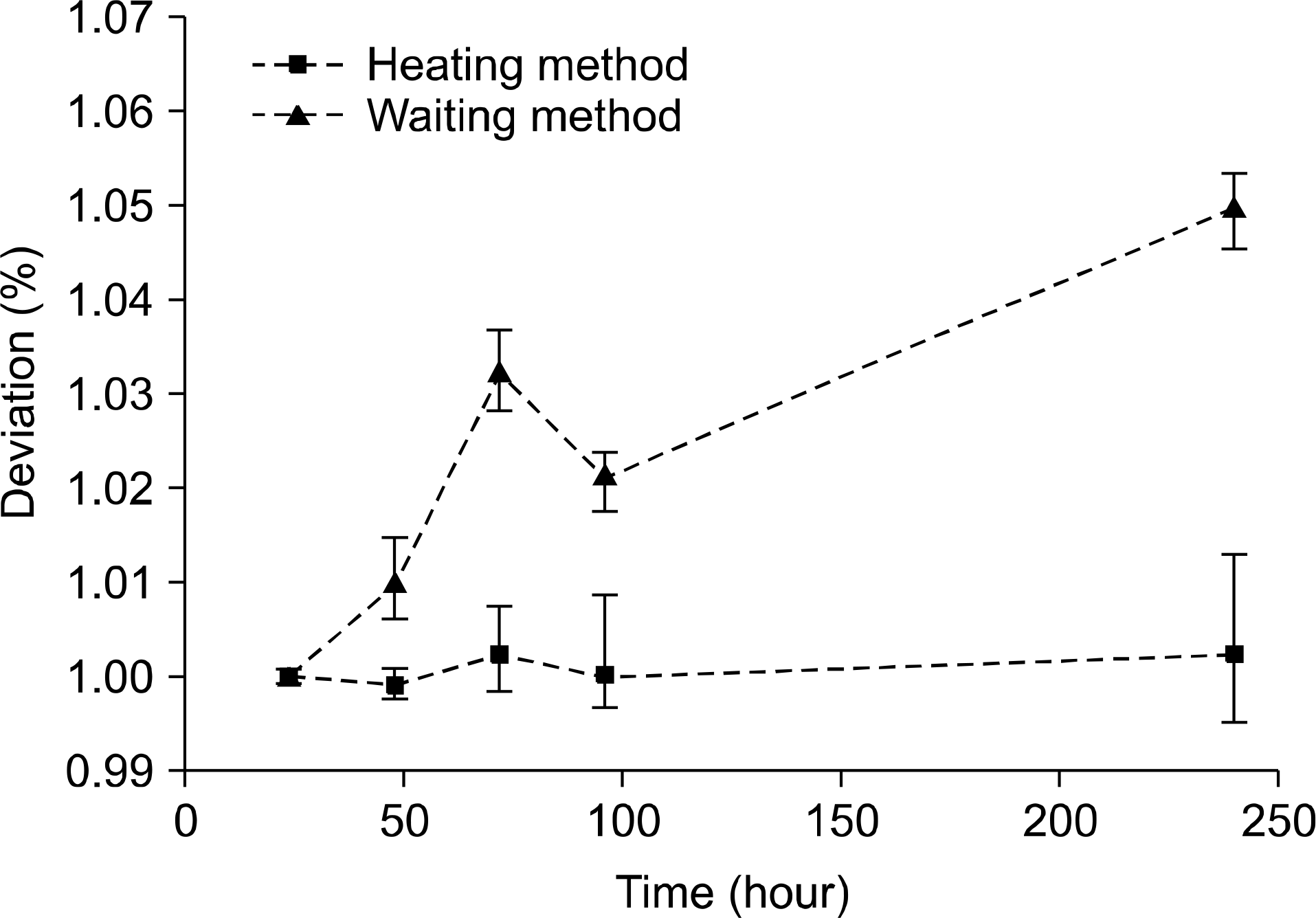 | Fig. 3.Mean reading deviations of GRDs according to time delay between pre-processing and reading. |
Table 1.
Patient numbers of different pre-processing methods and different sites.
| Site | Patient number | |
|---|---|---|
| Heating | Waiting | |
| Head | 4 | 16 |
| Neck | 29 | 25 |
| Breast | 23 | 26 |
| Chest | 9 | 3 |
| Abdomen | 36 | 25 |
| Pelvis | 25 | 30 |
| Total | 126 | 125 |
Table 2.
Comparison of GRD reading values with two different pre-processing methods.
Table 3.
Mean errors and deviations of different preprocessing methods and different sites.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download