Abstract
We estimated secondary scattered and leakage doses for intensity-modulated radiotherapy (IMRT), volumetric arc therapy (VMAT) and tomotherapy (TOMO) in patients with liver cancer. Five liver patients were planned by IMRT, VMAT and TOMO. Secondary scatter (and leakage) dose and organ equivalent doses (OEDs) are measured and estimated at various points 20 to 80 cm from the iso-center by using radiophotoluminescence glass dosimeter (RPLGD). The secondary dose per Gy from IMRT, VMAT and TOMO for liver cancer, measured 20 to 80 cm from the iso-center, are 0.01∼3.13, 0.03∼2.34 and 0.04∼1.29 cGy, respectively. The mean values of relative OED of secondary dose of VMAT and TOMO for five patients, which is normalized by IMRT, measured as 75.24% and 50.92% for thyroid, 75.14% and 40.61% for bowel, 72.30% and 47.77% for rectum, 76.21% and 49.93% for prostate. The secondary dose and OED from TOMO is relatively low to those from IMRT and VMAT. OED based estimation suggests that the secondary cancer risk from TOMO is less than or comparable to the risks from conventional IMRT and VMAT.
Go to : 
REFERENCES
1. 국가암정보센터. 암발생률 추세 분석. http://www.cancer.go.kr.
2. 국가암정보센터. 성별 10대암 조발생률. 2010. http://www.cancer.go.kr.
3. Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary & pancreatic diseases international: HBPD INT. 7(3):237–257. 2008.
4. Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio- frequency thermal ablation versus percutaneous ethanol injection. Radiology. 228(1):235–240. 2003.
5. Lin SM, Lin CJ, Lin CC, et al. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 127(6):1714–1723. 2004.
6. Cheng JC, Chuang VP, Cheng SH, et al. Local radiotherapy with or without transcatheter arterial chemoembolization for patients with unresectable hepatocellular carcinoma. International journal of radiation oncology, biology, physics. 47(2):435–442. 2000.

7. Hawkins MA, Dawson LA. Radiation therapy for hepatocellular carcinoma: from palliation to cure. Cancer. 106(8):1653–1663. 2006.
8. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 37(2):429–442. 2003.

9. Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. International Journal of Radiation Oncology, Biology, Physics. 21(1):109–122. 1991.

10. Liu MT, Li SH, Chu TC, et al. Three-dimensional conformal radiation therapy for unresectable hepatocellular carcinoma patients who had failed with or were unsuited for transcatheter arterial chemoembolization. Japanese Journal of Clinical Oncology. 34(9):532–539. 2004.

11. Giraud P, De Rycke Y, Dubray B, et al. Conformal radiotherapy (CRT) planning for lung cancer: analysis of intrathoracic organ motion during extreme phases of breathing. International Journal of Radiation Oncology, Biology, Physics. 51(4):1081–1092. 2001.

12. Fiveash JB, Hanks G, Roach M, et al. 3D conformal radiation therapy (3DCRT) for high grade prostate cancer: a multi- institutional review. International Journal of Radiation Oncology, Biology, Physics. 47(2):335–342. 2000.
14. Low DA, Mutic S. A commercial IMRT treatment-planning dose-calculation algorithm. International Journal of Radiation Oncology Biology Physics. 41(4):933–937. 1998.

15. Teh BS, Woo SY, Butler EB. Intensity modulated radiation therapy (IMRT): a new promising technology in radiation oncology. The Oncologist. 4(6):433–442. 1999.

16. Vaarkamp J, Krasin M. Reduction of target dose inhomogeneity in IMRT treatment planning using biologic objective functions. International Journal of Radiation Oncology Biology Physics. 49(5):1518–1520. 2001.

17. Ahamad A, Stevens CW, Smythe WR, et al. Promising early local control of malignant pleural mesothelioma following postoperative intensity modulated radiotherapy (IMRT) to the chest. Cancer J. 9(6):476–484. 2003.

18. Wieland P, Dobler B, Mai S, et al. IMRT for postoperative treatment of gastric cancer: covering large target volumes in the upper abdomen: a comparison of a step-and-shoot and an arc therapy approach. International Journal of Radiation Oncology Biology Physics. 59(4):1236–1244. 2004.

19. Malhotra HK, Raina S, Avadhani JS, deBoer S, Podgorsak MB. Technical and dosimetric considerations in IMRT treatment planning for large target volumes. Journal of Applied Clinical Medical Physics/American College of Medical Physics. 6(4):77–87. 2005.

20. Brahme A, Roos JE, Lax I. Solution of an integral equation encountered in rotation therapy. Physics in Medicine and Biology. 27(10):1221–1229. 1982.

21. Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Medical Physics. 35(1):310–317. 2008.

22. Yu CX. Intensity-modulated arc therapy with dynamic multileaf collimation: an alternative to tomotherapy. Physics in Medicine and Biology. 40(9):1435–1449. 1995.

23. Welsh JS, Patel RR, Ritter MA, Harari PM, Mackie TR, Mehta MP. Helical tomotherapy: an innovative technology and approach to radiation therapy. Technology in Cancer Research & Treatment. 1(4):311–316. 2002.

25. Cao D, Holmes TW, Afghan MK, Shepard DM. Comparison of plan quality provided by intensity-modulated arc therapy and helical tomotherapy. International Journal of Radiation Oncology Biology Physics. 69(1):240–250. 2007.

26. Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. International Journal of Radiation Oncology Biology Physics. 56(1):83–88. 2003.

27. Kim S, Min BJ, Yoon M, et al. Secondary radiation doses of intensity-modulated radiotherapy and proton beam therapy in patients with lung and liver cancer. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology. 98(3):335–339. 2011.

28. Howell RM, Hertel NE, Wang Z, Hutchinson J, Fullerton GD. Calculation of effective dose from measurements of secondary neutron spectra and scattered photon dose from dynamic MLC IMRT for 6 MV, 15 MV, and 18 MV beam energies. Medical Physics. 33(2):360–368. 2006.
29. Corporation A. RPL Glass Dosimeter/Small Element System Dose Ace. 2000.
30. Hsu SM, Yeh SH, Lin MS, Chen WL. Comparison on characteristics of radiophotoluminescent glass dosemeters and thermoluminescent dosemeters. Radiation Protection Dosimetry 119(1-4): 327-331. 2006.
31. KIM DW, Chung W. Characteristic study of radiophotoluminescence glass rod detector for clinical usages: skin and inner body in-vivo verification. J of Korean Phys Soc. 62(4):670–676. 2013.
Go to : 
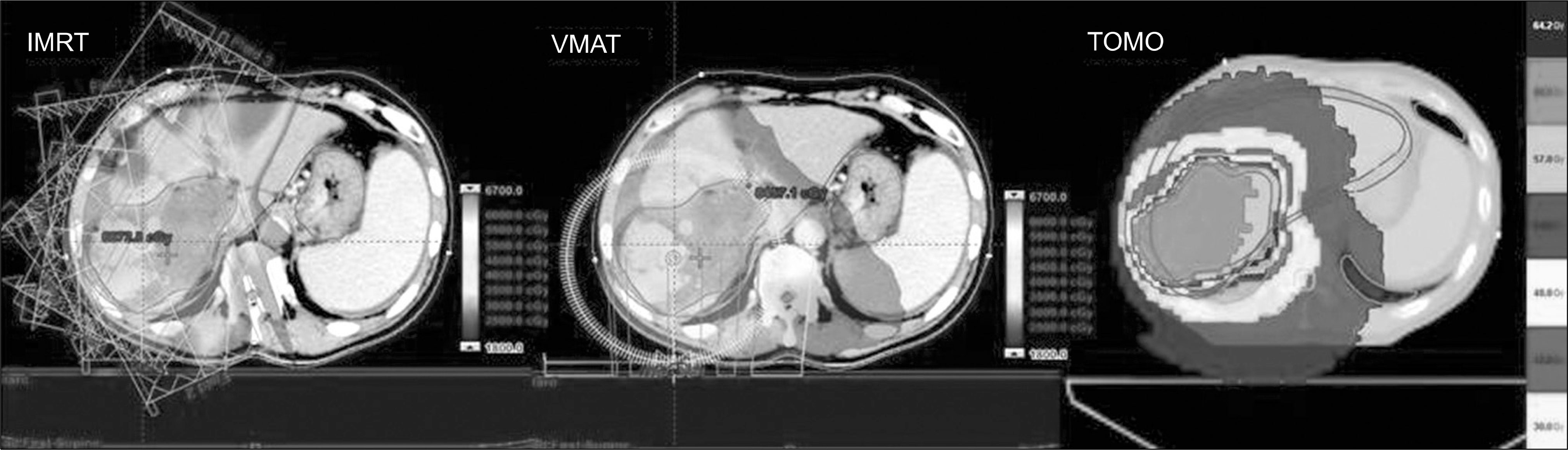 | Fig. 1.Treatment plan for patient #4 with different modalities; IMRT, VMAT and TOMO. The prescription dose 62.5 Gy with 25 fractions. |
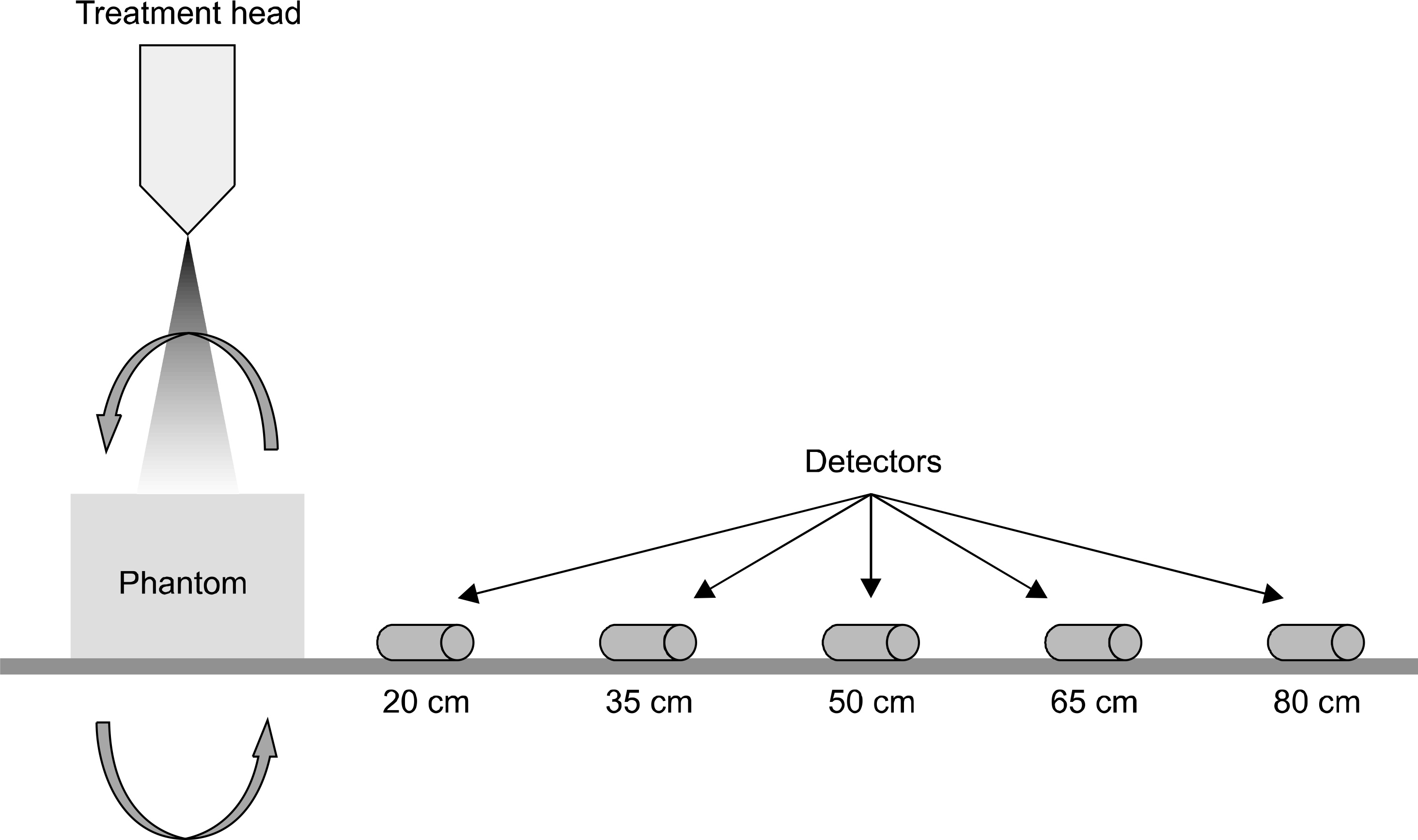 | Fig. 2.Secondary scattered dose measurement set up. Measuring positions are 20, 35, 50, 65 and 80 cm from the iso-center. Two RPLGDs are positioned at each measuring point. |
 | Fig. 3.The secondary scattered dose measurements of five patients for IMRT (diamond), VMAT (square) and TOMO (triangle) at each distance from the iso-center. All data are normalized by prescription dose. |
Table 1.
Patient information.
| ID | Sex | Age | Disease | Stage | PTV volume (cm3) |
|---|---|---|---|---|---|
| 1 | Male | 62 | Liver | III | 483 |
| 2 | Male | 54 | Liver | I | 60 |
| 3 | Male | 59 | Liver | III | 421 |
| 4 | Male | 49 | Liver | VI | 2,112 |
| 5 | Female | 42 | Liver | VI | 214 |
Table 2.
Treatment planning information.
Table 3.
At each points, the secondary scattered dose measurements as percentage of prescription dose.
Table 4.
Organ equivalent dose (Gy/GyRx) measurement per prescription dose at planning target volume.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download