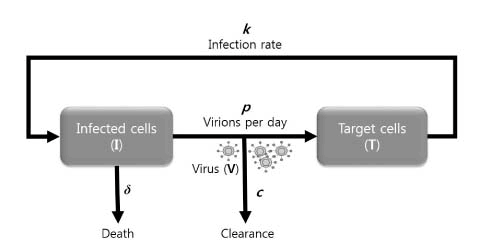
 | Fig. 1.Basic viral kinetic model. Infected cells (I), target cells (T) and virus (V) are the main components of viral kinetic model. New virions are produced at rate p, and die at rate δ. Free virions are cleared at rate c per virion. Although not shown, target cells are produced with rate λ and die with rate d per cell. |
Abstract
The importance of infection control has been increasingly recognized after the Middle East respiratory syndrome (MERS) outbreak in Korea. Infection management guidelines are based on viral pathway and kinetic studies. Viral kinetics is, in a broad sense, defined as the change rate of the virus over time after an infection and in a narrow sense, as a mathematical model that describes viral kinetics. Based on the results of these kinetic studies, the life cycle and viral infection can be understood and utilized to treat and manage infections. To control viral infection, viral shedding and its transmission should be properly understood. Classically, the mode of transmission can be divided into airborne, droplet, and contact transmissions. Based on viral shedding and transmission, the guidelines are established, which can be largely divided into standard, contact, droplet, and airborne precautions and isolation. For influenza virus or rhinovirus infection, standard and droplet precautions are applied. For parainfluenza virus or respiratory syncytial virus infection, standard and contact precautions are applied. For MERS coronavirus, standard, contact, and droplet precautions are required with isolation of patients and contacted people. Kindly check if suggested revision retained your intended meaning. The types of specimens widely varies, and due to mucus and different host immune status, the exact quantification is difficult. Despite these limitations, kinetic studies on respiratory viruses have been actively studied, and efforts to efficiently manage infections by conducting various studies are continuously required.
Go to : 
REFERENCES
1. Smith AM, Perelson AS. Influenza A virus infection kinetics: quantitative data and models. Wiley Interdiscip Rev Syst Biol Med. 2011; 3:429–445.

3. Jones RM, Brosseau LM. Aerosol transmission of infectious disease. J Occup Environ Med. 2015; 57:501–508.

4. Wei J, Li Y. Airborne spread of infectious agents in the indoor environment. Am J Infect Control. 2016; 44 9 Suppl:S102–S108.

5. Karim YG, Ijaz MK, Sattar SA, Johnson-Lussenburg CM. Effect of relative humidity on the airborne survival of rhinovirus-14. Can J Microbiol. 1985; 31:1058–1061.

6. Miller WS, Artenstein MS. Aerosol stability of three acute respiratory disease viruses. Proc Soc Exp Biol Med. 1967; 125:222–227.

7. Hall CB. The spread of influenza and other respiratory viruses: complexities and conjectures. Clin Infect Dis. 2007; 45:353–359.

8. KCDC. Guidelines for prevention and control of healthcare associated infections. Cheongju: KCDC; 2017.
9. Baccam P, Beauchemin C, Macken CA, Hayden FG, Perelson AS. Kinetics of influenza A virus infection in humans. J Virol. 2006; 80:7590–7599.

10. Breban R, Riou J, Fontanet A. Interhuman transmissibility of Middle East respiratory syndrome coronavirus: estimation of pandemic risk. Lancet. 2013; 382:694–699.

11. Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses. In: Knipe DM, Howley PM, editors. Fields virology. Philadelphia, PA: Wolters Kluwer Health; 2013. pp. 1186-1243.
12. Baek JH, Seo YB, Choi WS, Kee SY, Jeong HW, Lee HY, et al. Guideline on the prevention and control of seasonal influenza in healthcare setting. Korean J Med. 2014; 86:377–397.

13. Karron RA, Collins PL. Parainfluenza viruses. In: Knipe DM, Howley PM, editors. Fields virology. Philadelphia, PA: Wolters Kluwer Health; 2013. pp. 996-1023.
14. Siegel JD, Rhinehart E, book M, Chiarello L. Committee HICPA. In: 2007 Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. Atlanta, GA: Public Health Service, U.S. Department of Health and Human Services; 2007.
15. Collins PL, Karron RA. Respiratory syncytial virus and metapneumovirus. In: Knipe DM, Howley PM, editors. Fields virology. Philadelphia, PA: Wolters Kluwer Health; 2013. pp. 1086-1123.
16. Wright PF, Gruber WC, Peters M, Reed G, Zhu Y, Robinson F, et al. Illness severity, viral shedding, and antibody responses in infants hospitalized with bronchiolitis caused by respiratory syncytial virus. J Infect Dis. 2002; 185:1011–1018.

17. Gern JE, Palmenberg AC. Rhinoviruses. In: Knipe DM, Howley PM, editors. Fields virology. Philadelphia, PA: Wolters Kluwer Health; 2013. pp. 531-549.
18. Mosser AG, Vrtis R, Burchell L, Lee WM, Dick CR, Weisshaar E, et al. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med. 2005; 171:645–651.

19. Hall CB, Douglas RG Jr, Geiman JM. Respiratory syncytial virus infections in infants: quantitation and duration of shedding. J Pediatr. 1976; 89:11–15.

20. Min CK, Cheon S, Ha NY, Sohn KM, Kim Y, Aigerim A, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 2016; 6:25359.

21. Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013; 369:407–416.

22. Choi S, Jung E, Choi BY, Hur YJ, Ki M. High reproduction number of Middle East respiratory syndrome coronavirus in nosocomial outbreaks: mathematical modelling in Saudi Arabia and South Korea. J Hosp Infect. 2018; 99:162–168.

23. Zhang XS, Pebody R, Charlett A, de Angelis D, Birrell P, Kang H, et al. Estimating and modelling the transmissibility of Middle East Respiratory Syndrome CoronaVirus during the 2015 outbreak in the Republic of Korea. Influenza Other Respir Viruses. 2017; 11:434–444.

24. Korea Centers for Disease Control and Prevention (KCDC). 2017 MERS response guidelines. Cheongju: KCDC; 2017.
25. Chen SC, Chio CP, Jou LJ, Liao CM. Viral kinetics and exhaled droplet size affect indoor transmission dynamics of influenza infection. Indoor Air. 2009; 19:401–413.

26. Sung H. Kinetics and infection control of respiratory viruses; Korean Journal of Nosocomial Infection Control, the 21st Conference; 2016 May 27; Seoul, Korea.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download