Abstract
Spinal cord infarction (SCI) is a rare but often disastrous disorder caused by various pathologic status. The research reports a 34-year-old woman developed SCI after spinal anesthesia for relieving labor pain and total hysterectomy, due to postpartum hemorrhage. Three times of spinal anesthesia were performed, two for controlling labor pain, which were succeeded and one for cesarean delivery, which was failed. After delivery, she was referred to Dankook University Hospital because of uterine atony. On arrival in the emergency room, the blood pressure decreased to 58/32 mmHg, she had a pulse rate of 144 beats/min. While emergent total hysterectomy was performed, she remained hemodynamically stable and there was no episode of hypotension. Since she complained of immobility and loss of sensations the day after the operation, magnetic resonance imaging was performed and showed ischemic change at central portion of the spinal cord at T8 to L1 level.
Acute spinal cord infarction (SCI) is a rare cause of acute myelopathy. Although incidence is not perfectly clear because of its rarity, one study noted SCI accounted for 1.2% of all stroke admissions.1 There are various etiologies of SCI, including arteriosclerosis, aortic surgery and stenting, aortic dissection, vertebral artery dissection and occlusion, vasculitis, and systemic hypotension. A big portion of these etiologies remains unclear.2 Patients typically present with acute back or neck pain at the onset of symptoms, and areflexic flaccid paraplegia or tetraplegia with loss of bowel and bladder continence.3 The research reports a 34-year-old woman developed spinal cord infarction after total hysterectomy, due to severe postpartum hemorrhage.
A 34-year-old woman was referred for postpartum hemorrhage, after cesarean delivery for prolonged deceleration phase at a primary health care center. She had no remarkable past medical history including vascular risk factors such as hypertension, diabetes mellitus, and hyperlipidemia. During labor, she had two times of spinal anesthesia to control labor pain and those were effective. However, emergent cesarean delivery was decided due to prolonged deceleration phase. One more time of spinal anesthesia for the operation was tried, but additional general anesthesia had to be performed, since she continuously complained of pain. Even though cesarean delivery was uneventfully performed, she was referred to Dankook University Hospital 1 hour after the delivery because of postpartum hemorrhage caused by uterine atony.
On arrival in the emergency room, the blood pressure (BP) was 118/76 mmHg. However, a few minutes later, it decreased to 58/32 mmHg, and the pulse rate was 144 beats/min. The hemoglobin (HB) and hematocrit (HCT) levels were 7.8 g/dL and 22.7%. respectively. After Hartman solution and a bolus of 0.3 mg of epinephrine was given, BP immediately returned to 154/78 mmHg. However, since bleeding was continued, BP decreased again to 62/42 mmHg and 60/38 mmHg, so that a bolus of 0.3 mg of epinephrine was given each time. Five pints of packed red blood cells (p-RBC) were also transfused. Within 35 minutes after arriving at the emergency room, she was sent to the operating room for emergent total abdominal hysterectomy. During the operation, additional five pints of p-RBC and six pints of fresh frozen plasma (FFP) were transfused. She remained hemodynamically stable and there was no episode of hypotension during the operation. She was sent to the intensive care unit for close observation. An hour after the operation, HB level, HCT and platelet count were 7.7 g/dL, 22.4% and 49,000/µL. Four pints of p-RBC, four pints of FFP, and eight pints of platelet concentrate were transfused. Because HB level was 8.1 g/dL even after the transfusion, two more pints of p-RBC and FFP were transfused, respectively.
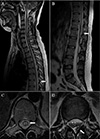
The following day, HB level, HCT and platelet count were 9.1 g/dL, 27.1% and 76,000/µL. In the afternoon, she started complaining of motor weakness and abnormal sensation in her both legs. She had little right big toe flexor movement (grade 1 out of 5), and could not move other parts of both legs as well. Also, she had decreased, almost absent, proprioception, pinprick, and touch sensation to L1. When she was pinched her legs as hard as possible she had little feeling of numbness. Initial clinical impression was slow spinal block regression. However, neurologic examination remained nearly unchanged until one day later, an unenhanced magnetic resonance imaging (MRI) of the whole spine was performed. It demonstrated long segmental signal change of the spinal cord at T8 to L1 level, which suggested ischemic change more likely than inflammation or demyelinating myelopathy, and posterior epidural hematoma at L1 to L2 level, probably due to spinal anesthesia (Fig. 1).
During the next week, the patient's motor and sensory function did not improve, but deteriorated. Her motor strength of entire leg from hip to toe was 0 out of 5, and she had no touch, pinprick, vibration sense, and proprioception. The patient's urinary catheter was removed on post-operative day 9, and it was discovered that she had no sensation to void. Moreover, her bowel function was not normal, since she had no sensation to defecate and had fecal incontinence.
The patient was transferred to rehabilitation medicine 1 week postoperatively for ongoing physiotherapy. On admission to the rehabilitation unit, she was independent for her activities of daily living, except voiding and defecating, and was ambulating in a wheelchair. She was transferred to a rehabilitate hospital 1 month later for more specialized therapy. At 2 months' follow-up, her right ankle and proximal hip strength was improved to 3 out of 5 and 2 out of 5, respectively. She still had loss of sensory below L1, and bladder and bowel incontinence. At 3 months' follow-up, her right and left knee extension strength was improved to 4 and 3 out of 5, respectively. Her rehabilitation therapy is still ongoing, so that further follow-up is needed.
Anatomically, the spinal cord is perfused by 3 main arteries-a single anterior spinal artery (ASA) supplies the anterior twothirds of the cord and 2 posterior spinal arteries (PSA) supply posterior one-third. There are 4 to 8 radicular arteries that feed into ASA. The largest and most important radicular artery is the artery of Adamkiewicz, which mostly arises from the aorta between T9 and T12. It supplies the lower thoracic and lumbar cord. There are few anterior radicular arteries and caliber of ASA is significantly attenuated, making thoracic lesions that particularly vulnerable to infarction, during global hypoperfusion.45 Since there are more radicular arteries that feed PSA, vascular supply to PSA is more reliable. As a result, posterior spinal cord infarction is seen less frequently.3
The neurologic presentation of spinal cord infarction is generally defined by the vascular territory involved. Because ASA supplies corticospinal and spinothalamic tracts, the ASA syndrome is typically characterized by an areflexic flaccid paraplegia or tetraplegia with a dissociated sensory deficit-impaired pain and temperature with preserved proprioception. The autonomic system is usually influenced and bowel and bladder incontinence is in most patients. The PSA syndrome includes the loss of proprioceptive and vibratory sense below the level of the lesion. The central cord infarction occurs after cardiac arrest of prolonged hypotension. It is characterized by bilateral spinothalamic deficits with sparing of the posterior column. The transverse infarction is manifested by bilateral motor deficits and complete sensory loss involving all modalities. 367 The patient in this case was close to the transverse infarction.
Mechanisms that cause spinal cord ischemia vary, including arteriosclerosis, aortic surgery and stenting, aortic dissection, vertebral artery dissection and occlusion, vasculitis, and systemic hypotension, but a big portion of it remains unclear.2 The etiology of this case has not been confirmed. Arteriosclerosis seems less likely to be contributory in this case, given the young age and negative relevant cardiac history. Also, no evidence of atherosclerotic plaque rupture and vasculitis refutes the possibility of occlusion caused by embolization. Mechanical compression of the aorta may also be a cause, but cesarean delivery is not a surgery that is concerned a risk for compression of it for a prolonged time.
Even though the duration seems to be short, hypovolemia could be a cause of the ischemia. Rao Shailaja et al.8 reported a very similar case, where a woman suffered from severe postpartum hemorrhage causing systemic hypotension leading to spinal cord infarction. There is no comment on whether neuraxial anesthesia was used. MRI showed ischemia from medulla to C7 level, and neurological examination showed spastic quadriparesis with grade 3 out of 5.
The complication of spinal cord infarction after neuraxial anesthesia in the obstetrical population is rare. In a retrospective series of more than 500,000 obstetric population in the United Kingdom, one patient was found to have had anterior spinal artery syndrome after neuraxial anesthesia. The cause of it was not confirmed.9 However, since the researchers do not know exactly what kinds of drug were used or doses of drugs, the possibility cannot be ruled out.
Soda et al.10 presented a case of a woman, who was found to have thrombocytosis and high fibrinolytic activity presenting with acute paraparesis, sensory disturbance in lower extremity, and urinary disturbance 6 days postpartum. She improved after administration of antithrombotic agents and methylpredonisolone. Hypercoagulable state of pregnancy may able cause spinal cord ischemia in this case.
Treatment is depending on the level and severity of SCI. These include airway and ventilation management, hemodynamic stabilization, fever and glycemic control. And to address and correct the underlying mechanism of injury, there are few approved measures such as antiplatelet therapy, systemic thrombolysis, or mechanical thrombectomy.311 However, the use of antiplatelet treatment is recommended only in patients with underlying vascular risk factors or comorbid vascular disease. Thrombolytic therapy has been used effectively in a few cases, but there is still a controversy over its efficacy.11 Moreover, since the patient in this case was suffered from severe postpartum hemorrhage, there would be increased risk of bleeding if thrombolysis was performed.
In conclusion, the researchers cannot exactly verify the etiology in this present case, but hypothesize that the combiation of hypovolemia, a hypercoagulable state, and probably spinal anesthesia may have all contributed to spinal cord infarction. Although rare, SCI should be considered in any postpartum women, who present with neurological deficit. It is important to recognize SCI acutely and address the potential cause to correct the underlying mechanism of injury.
Figures and Tables
Fig. 1
(A, B) Sagittal T2-weighted MRI image of the whole spine, showing long segmental signal change of the spinal cord at T8 (arrow in A) to L1 level (arrow in B). (C) Axial T2-weighted MRI image at L11 vertebral level showing marked central T2 prolongation and swelling of the spinal cord (arrow), consistent with acute/subacute ischemia. (D) Axial T2-weighted MRI image at L1-2 vertebral level showing posterior epidural hematoma (arrow). MRI, magnetic resonance imaging.
References
1. Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine (Baltimore). 1989; 68:282–292.
2. Weidauer S, Nichtweiß M, Hattingen E, Berkefeld J. Spinal cord ischemia: aetiology, clinical syndromes and imaging features. Neuroradiology. 2015; 57:241–257.

3. Vongveeranonchai N, Zawahreh M, Strbian D, Sundararajan S. Evaluation of a patient with spinal cord infarction after a hypotensive episode. Stroke. 2014; 45:e203–e205.

5. Colman MW, Hornicek FJ, Schwab JH. Spinal cord blood supply and its surgical implications. J Am Acad Orthop Surg. 2015; 23:581–591.

6. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006; 63:1113–1120.
7. Vargas MI, Gariani J, Sztajzel R, Barnaure-Nachbar I, Delattre BM, Lovblad KO, et al. Spinal cord ischemia: Practical imaging tips, pearls, and pitfalls. AJNR Am J Neuroradiol. 2015; 36:825–830.

8. Rao Shailaja V, Anjali P, Borkar MS, Gajanan S, Vimlesh P. A rare complication of pre-eclampsia and severe postpartum hemorrhage-spinal cord leading to anterior spinal artery syndrome. IOSR-JDMS. 2015; 14:38–40.
9. Scott DB, Hibbard BM. Serious non-fatal complications associated with extradural block in obstetric practice. Br J Anaesth. 1990; 64:537–541.

10. Soda T, Shimizu I, Takagaki M, Yamada K, Dehara M, Teramoto Y, et al. A case of benign postpartum anterior spinal artery syndrome with thrombocytosis and high fibrolytic activity. Brain Nerve. 2011; 63:1125–1129.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download