Abstract
Susceptibility-weighted imaging (SWI) is well known for detecting the presence of hemorrhagic transformation, microbleeds and the susceptibility of vessel signs in acute ischemic stroke. But in some cases, it can provide the tissue perfusion state as well. We describe a case of a patient with hyperacute ischemic infarction that had a slightly hypodense, patchy lesion at the left thalamus on the initial SWI, with a left proximal posterior cerebral artery occlusion on a magnetic resonance (MR) angiography and delayed time-to-peak on an MR perfusion performed two hours after symptom onset. No obvious abnormal signals at any intensity were found on the initial diffusion-weighted imaging (DWI). On a follow-up MR image (MRI), an acute ischemic infarction was seen on DWI, which is the same location as the lesion on SWI. The hypointensity on the initial SWI reflects the susceptibility artifact caused by an increased deoxyhemoglobin in the affected tissue and vessels, which reflects the hypoperfusion state due to decreasing arterial flow. It precedes the signal change on DWI that reflects a cytotoxic edema. This case highlights that, in some hyperacute stages of ischemic stroke, hypointensity on an SWI may be a finding before the hyperintensity is seen on a DWI.
An ischemic stroke is one of the most commonly occurring diseases worldwide with a high mortality rate and high rates of disability. Therefore, early detection of an acute stroke is extremely important (1). Among magnetic resonance (MR) techniques, diffusion-weighted imaging (DWI) is particularly sensitive to detect an acute ischemic stroke with high diagnostic accuracy. And T2/T2*-weighted imaging, perfusion-weighted imaging (PWI), and MR angiography (MRA) have been incorporated into the initial assessment of acute stroke (1). However, there have been negative DWI results in the hyperacute stage of ischemic stroke. It is possible that during some ischemic strokes, there is a reduction in blood flow severe enough to cause symptoms, but not severe enough to cause a DWI lesion that shows cellular edema (2). In these instances, susceptibility-weighted imaging (SWI) is not only sensitive enough to detect hemorrhagic transformations in an infarcted area or the susceptibility signs of vessels. In some cases SWI can provide perfusion information shown as a hypointense signal change in a hypoperfused area called the SWI-DWI mismatch (3).
We present a rare case of acute embolic infarction that revealed an earlier hypointense signal change of the infarcted area on a SWI before any change on a DWI.
A 58-year-old man was referred to our emergency department with notable mental changes, an initial National Institutes of Health Stroke Scale (NIHSS) score of 26, a modified Rankin Scale (mRS) score of 5, and a Glasgow Coma Scale (GCS) score of 6. The patient's past medical history included a percutaneous coronary intervention on the left anterior descending coronary artery due to a myocardial infarction three months ago. Brain computed tomography (CT), DWI, MRA, MR perfusion, and SWI were performed two hours after symptom onset. MRI was performed with a Philips 3T Achieva scanner (Philips Medical Systems, Best, the Netherlands) by using a 16 channel head coil. SWI sequence was obtained in the axial plane using a three-dimensional gradient echo with repetition/echo time 22/32 ms, slice thickness 3 mm, flip angle 10° and a matrix of 308 × 309 pixels. No evidence of brain hemorrhage was seen on the initial brain CT, and no obvious abnormal signal intensities were found on the initial DWI. However, SWI showed a small, slightly hypointense, patchy lesion at the left thalamus, and a left proximal posterior cerebral artery occlusion on the MRA, and delayed time-to-peak on a MR perfusion (Fig. 1).
The patient was hospitalized and an intravenous tissue plasminogen activator and oral dual antiplatelet therapy were administered. Follow-up brain imaging, including a CT and an MRI, was taken two days later. An acute ischemic infarction was seen on the DWI and T2-FLAIR (fluid attenuated inversion recovery) images in the left thalamic area, which is the same location as the hypointense lesion seen on the SWI in the initial exam (Fig. 2). Hemorrhagic transformation of the infarcted area was also noted on brain MR T2* gradient echo sequences (T2*-GRE) (Fig. 2). We changed the patient's oral treatment from dual antiplatelet therapy to monotherapy treatment with aspirin. After two weeks of treatment, the patient's NIHSS score improved to a 3, and the mRS score improved to a 2. In addition, there was no progression of hemorrhagic transformation.
SWI is a high resolution, three-dimensional, gradient echo T2* MRI technique that is based on the blood oxygen-dependent (BOLD) image principle (4). It uses the magnetic susceptibility effects caused by local inhomogeneities of the magnetic field (5). Therefore, SWI can offer information about any tissue that has local susceptibility changes, such as deoxygenated blood, hemosiderin and ferritin (1). In hypoperfused brain tissue during acute ischemic stroke, an increase of deoxyhemoglobin levels and a decrease of oxyhemoglobin levels in tissue capillaries and draining veins may generate hypointensity on an SWI (5).
In our case, the SWI showed a small, hypointense lesion in the acute ischemic area earlier than a signal change on the DWI. Although these imaging findings have rarely been described, we postulate the hypointensity on SWI reflects the susceptibility artifact caused by increased deoxyhemoglobin in the affected tissue and vessels. An increase of deoxyhemoglobin, which reflects the hypoperfusion state due to decreasing arterial flow, precedes the signal change on DWI that reflects a cytotoxic edema (3). Previous studies have revealed that a mismatch between a smaller DWI cytotoxic edema and a larger SWI hypointense area implied that SWI has the capability to predict stroke evolution by reflecting the tissue perfusion state (3). This case might be an extreme instance to apply the previous concept because DWI did not show any initial signal change.
Hemorrhagic transformation of the infarcted area clearly occurred on follow-up imaging two days later. It is well known that SWI is much more sensitive for the detection of hemorrhagic transformation than both CT and T2* gradient echo images (1). In this regard, hypointensity on SWI may have a predictive value for hemorrhagic transformation related to the severe hypoperfusion state with more deoxyhemoglobin or blood-brain barrier break. However, it should be investigated further in future studies.
Undoubtedly, DWI is currently the most sensitive method for the detection of acute ischemic infarction (2). However, in some instances, hypointensity on SWI may show an earlier finding of ischemic stroke before the hyperintensity is seen on the DWI during the hyperacute stage.
Figures and Tables
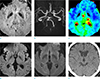 | Fig. 1Initial CT and MRI two hours after the onset of ischemic infarction. Susceptibility-weighted imaging showed a slight hypointensity at the left thalamus (a) with left posterior cerebral artery occlusion (b), and delayed time-to-peak (c). However, no obvious abnormal signal changes were shown on diffusion-weighted imaging (d), and T2-FLAIR (fluid attenuated inversion recovery) (d). On the brain CT, there was no evidence of brain hemorrhage (f). |
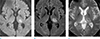 | Fig. 2Follow-up MRI after two days. Acute ischemic infarction at the left thalamus showed hyperintensity on diffusion-weighted imaging (a), and T2-FLAIR (fluid attenuated inversion recovery) (b). Additionally, petechial hemorrhagic transformation in the infarcted area was noted on T2* gradient echo sequences (c). |
References
1. Leiva-Salinas C, Wintermark M. Imaging of acute ischemic stroke. Neuroimaging Clin N Am. 2010; 20:455–468.

2. Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Clinically confirmed stroke with negative diffusion-weighted imaging magnetic resonance imaging: longitudinal study of clinical outcomes, stroke recurrence, and systematic review. Stroke. 2015; 46:3142–3148.
3. Fujioka M, Okuchi K, Iwamura A, Taoka T, Siesjo BK. A mismatch between the abnormalities in diffusion- and susceptibility-weighted magnetic resonance imaging may represent an acute ischemic penumbra with misery perfusion. J Stroke Cerebrovasc Dis. 2013; 22:1428–1431.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download