Abstract
Hepatic toxocariasis is a type of visceral larva migrans caused by the migration of second-stage larvae of certain nematodes such as Toxocara canis to the liver. Histologically, the condition is characterized by granulomatous lesions containing eosinophils and inflammatory cells. We report a case of hepatic toxocariasis with atypical clinical and radiologic findings presenting as distinct, solitary hepatic nodule detected in a middle-aged woman.
Hepatic toxocariasis is a form of visceral larva migrans (VLM) caused by migration of second-stage larvae of certain nematodes such as Toxocara canis (T. canis) to the liver. Hepatic toxocariasis typically involves granulomatous lesions containing eosinophils and inflammatory cells (1). Computed tomography (CT) and magnetic resonance imaging (MRI) findings usually show multiple, ill-defined, oval lesions, measuring 1.0–1.5 cm in diameter (2). We have recently experienced a case of hepatic toxocariasis that presented as an appearance different from typical radiologic features. Therefore, we report on a case of hepatic toxocariasis with a radiologic-pathologic correlation.
A 59-year-old woman was admitted to our hospital following a complaint of left flank discomfort and dyspepsia for 2 weeks. She had undergone balloon angioplasty for angina pectoris 3 years ago. She reported weight loss of 1.5 kg in 1 month. The patient had a dog, but she had no history of ingesting uncooked meat or cow's liver, nor had she traveled abroad in recent years. The physical examination on admission was normal, with no hepatosplenomegaly or lymphadenopathy. Her vital signs were within the normal range. Blood cell count at admission was normal (5200 leukocytes/mm3 with 3.5% eosinophils). Blood chemistry findings were also within the normal range.
A 2-phase abdominal contrast-enhanced CT performed at an outside hospital revealed a relatively well-defined, low attenuation 0.5-cm nodular lesion on the dome of the right hepatic lobe (Fig. 1). The lesion had a suspicious rim enhancement after injection of the contrast media. The diagnoses suggested by the appearance included a simple cyst with a partial volume effect, an inflammatory lesion, or malignancy. However, it was too small for a definitive identification.
Twenty days after the CT, the patient underwent a liver MRI to characterize the tiny lesion. The lesion was tiny and had a faint low-signal intensity on a T1-weighted axial image (Fig. 2a) and a bright signal intensity on a T2-weighted axial image (Fig. 2b). It had a high signal intensity on a diffusion-weighted image, with a b-value of 800 (Fig. 2c), and a low value on the apparent diffusion coefficient (ADC) map (Fig. 2d), suggesting diffusion restriction. On gadoxetic acid (GD-EOB-DTPA)-enhanced dynamic MRI, the appearance of the lesion was hypointense in the arterial (Fig. 2e), portal venous (Fig. 2f), equilibrium (Fig. 2g), and hepatobiliary phases (Fig. 2h). The lesion was best seen in the portal venous phase and showed a definite rim enhancement in the arterial phase. The radiologic differential diagnosis included a complicated cyst, abscess, atypical hemangioma, and metastasis.
After 6 months, repeat 2-phase abdominal CT revealed a well-circumscribed, round, low attenuation lesion with a diameter that expanded from 0.5 cm to 1.5 cm (Fig. 3). The attenuation of the lesion is 35 Hounsfield unit (HU) in the pre-contrast phase and 31 HU in the portal venous phase. Her symptoms of left flank discomfort and dyspepsia were relieved. There was no further weight loss. The whole blood cell count showed 6440 leukocytes/mm3 and 0.6% eosinophils, which were still within the normal range. Based on the CT and MRI findings, options for differential diagnoses included a complicated cyst, indolent abscess, metastasis, or a parasitic infection.
Because of the increased size of the lesion, we performed an ultrasound-guided biopsy of the hepatic nodule. The lesion was round and slightly hypoechoic on ultrasound (Fig. 4). Pathological findings were indicative of hepatocyte necrosis, surrounded by a granulomatous inflammatory reaction, consisting predominantly of eosinophils and multinucleated giant cells (Fig. 5). Although the remnants of T. canis larvae were not identified, the findings were suggestive of an eosinophilic abscess caused by a parasitic infection such as toxocariasis. An IgG enzyme-linked immunosorbent assay (ELISA) using a T. canis larvae antigen was positive at an absorbance of 1.459 (cut off: 0.920). Therefore, we made a final diagnosis of hepatic toxocariasis.
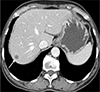
The patient was given 400 mg of albendazole twice a day for 3 weeks. After 5 months, the follow-up ELISA was still positive. The last follow-up, using 3-phase abdominal dynamic CT, was performed 2.5 years later and indicated that the lesion had decreased from 1.5 to 1.0 cm, without a change in location or any new hepatic lesion (Fig. 6). The nodule is best seen in the portal venous phase.
This is an atypical case of hepatic toxocariasis, in that it manifested as a distinct, single hepatic nodule without peripheral eosinophilia.
The term, toxocariasis, refers to human infection by the larvae of T. canis or T. cati (2). VLM occurs when the second-stage larvae of the organism migrate through the gut wall and lodge in various organs. The condition in the liver is characterized by granulomatous lesions containing eosinophils and inflammatory cells (1). VLM secondary to T. canis is primarily a disease of children who live in poor sanitary conditions, and are likely to ingest contaminated soil containing T. canis eggs deposited by dogs infested with the parasite (3). The most common route of infection in adult humans, however, is through ingestion of raw liver from a cow (4). Regardless of the source, the eggs are swallowed and hatched in the intestine. They may then pass through the intestinal wall into the portal vein, reaching the liver (5). In our patient, however, the source of the parasite could not be definitively proven.
Common signs and symptoms of hepatic toxocariasis include fever, abdominal pain, and hepatomegaly (6). However, our patient's symptoms of left flank discomfort and dyspepsia are nonspecific and did not correlate with imaging findings.
ELISA is helpful in the diagnosis, as it can detect human IgG antibodies against Toxocara excretory/secretory antigens (2). Laboratory evaluation of patients with toxocariasis almost always reveals leukocytosis with notable eosinophilia (6). Peripheral eosinophilia was not, however, found in our patient at any time during her course of treatment.
CT findings reflect pathological findings. The slow migration of T. canis larvae often results in a periportal eosinophilic infiltrate, abscess, or granuloma. The lesions usually appear as multiple small, ill-defined, oval or elongated, low attenuation nodules on dynamic CT in the portal venous phase (378). Eosinophilic abscesses may, in some cases, appear as persistent low-attenuation lesions in the arterial, portal venous, and equilibrium phases (2). On follow-up imaging, the lesions may improve, or their positions may change (4). Our case is interesting for the following reasons: First, the initial CT revealed a tiny, relatively distinct, solitary hepatic nodular lesion, which, we believe, indicated the early stage of hepatic VLM. Second, on follow-up, while the lesion increased in size, there was no change in location or shape, nor did we see new lesions. Third, the lesion had a well-defined margin and was spherical on the follow-up CT.
According to previous reports of MRI in VLM, the lesions have faint, low-signal intensities on T1-weighted images and faint high-signal intensities on T2-weighted images. VLM nodules are usually hyperintense on diffusion-weighted images, with a b value of 1000 and an associated restriction on ADC maps (9). Most focal lesions are poorly defined on gadolinium-enhanced, dynamic MRI, with low-signal intensities in the portal venous phase, but isointense or nearly isointense in the arterial and equilibrium phases (9). Eosinophilic abscesses may appear as nodular-enhancing lesions or low-intensity nodules with rim enhancements (2). However, Mukund et al. (1) suggested that variable MRI findings are seen, depending on the age of the lesions and the amount of protein contained within the abscess. In our case, MRI findings revealed a tiny, distinct hepatic nodule in the arterial, portal venous, equilibrium, and the hepatobiliary phase, which had a definite rim enhancement in the arterial phase. Moreover, because the lesion had a bright signal intensity on the T2-weighted image, the complicated cyst had to be considered in a differential diagnosis. However, in terms of pathology, the MRI findings likely reflected liquefaction of an eosinophilic abscess.
Lastly, VLM nodules are reported as non-spherical lesions with fuzzy margins, with no or insignificant rim enhancement, which helps to differentiate them from metastases (210). Although she had no history of cancer, we had to consider metastasis in our differential diagnosis, based on the appearance of the patient's nodule i.e., round and with a definite rim enhancement on the MRI.
In summary, we present an atypical case of hepatic toxocariasis in a middle-aged woman. Neither imaging nor laboratory findings were suggestive of this diagnosis. When the lesion was eventually biopsied, the findings were consistent with a parasitic infection, but the definitive diagnosis could only be made solely on serology. This report serves to remind radiologists of this rare entity and the fact that it may present an appearance different from the typical radiologic features of hepatic VLM.
Figures and Tables
Fig. 1
Hepatic toxocariasis in a 59-year-old woman, CT findings. Contrast-enhanced abdominal CT at the level of the hepatic dome displays a tiny, relatively well-defined nodular lesion (arrow) with possible peripheral enhancement.
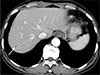
Fig. 2
Hepatic toxocariasis in a 59-year-old woman, MRI findings. (a) T1-weighted axial MR image shows a tiny, faintly hypointense nodule. (b) T2-weighted axial MR image shows a tiny, distinct nodule with bright signal intensity. (c) Diffusion-weighted image with a b value of 800 shows a hyperintense nodule. (d) The corresponding lesion is hypointense on the ADC map, representing diffusion restriction. (e-g) Gadolinium-enhanced T1-weighted images show a hypointense nodule with significant rim enhancement in the arterial phase (e) and with insignificant rim enhancement in the portal venous (f) and equilibrium (g) phases. (h) The hepatobiliary phase image shows hypointense nodule without rim enhancement. The arrow indicates the lesion in each image.
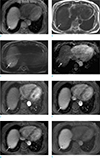
Fig. 3
Follow-up CT (6 months later). 2-phase abdominal CT image in the portal venous phase reveals a round, well-defined nodule (arrow) 1.5 cm in size with an attenuation of 31 Hounsfield unit.
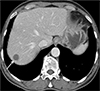
Fig. 4
Hepatic toxocariasis in a 59-year-old woman, ultrasound findings. Ultrasound of the liver shows a round, slightly hypoechoic nodule (arrow) on the right hepatic dome.
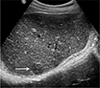
Fig. 5
Hepatic toxocariasis in a 59-year-old woman, microscopic findings. Photomicrograph (Hematoxylin & Eosin, × 40) reveals hepatocyte necrosis (N) surrounded by a granulomatous inflammatory reaction consisting predominantly of eosinophils (arrowheads) and a multinucleated giant cell (arrow), suggestive of parasitic infection. The remnants of Toxocara canis larvae were not identified.
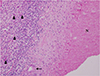
Fig. 6
Follow-up CT (2.5 years later). Two and a half years after treatment for Toxocara canis infection was begun, 3-phase abdominal dynamic CT image in the portal venous phase reveals a 1.0-cm lesion (arrow), which had decreased size from 1.5 cm at the beginning of treatment. The lesion's position has no changed, nor are there any new hepatic lesions.
References
1. Mukund A, Arora A, Patidar Y, et al. Eosinophilic abscesses: a new facet of hepatic visceral larva migrans. Abdom Imaging. 2013; 38:774–777.

3. Ishibashi H, Shimamura R, Hirata Y, Kudo J, Onizuka H. Hepatic granuloma in toxocaral infection: role of ultrasonography in hypereosinophilia. J Clin Ultrasound. 1992; 20:204–210.

4. Chang S, Lim JH, Choi D, et al. Hepatic visceral larva migrans of Toxocara canis: CT and sonographic findings. AJR Am J Roentgenol. 2006; 187:W622–W629.
5. Dupas B, Barrier J, Barre P. Detection of Toxocara by computed tomography. Br J Radiol. 1986; 59:518–519.
6. Arango CA. Visceral larva migrans and the hypereosinophilia syndrome. South Med J. 1998; 91:882–883.

7. Bhatia V, Batra Y, Acharya SK. Prophylactic phenytoin does not improve cerebral edema or survival in acute liver failure--a controlled clinical trial. J Hepatol. 2004; 41:89–96.

8. Hayashi K, Tahara H, Yamashita K, et al. Hepatic imaging studies on patients with visceral larva migrans due to probable Ascaris suum infection. Abdom Imaging. 1999; 24:465–469.

9. Laroia ST, Rastogi A, Sarin S. Case series of visceral larva migrans in the liver: CT and MRI findings. Int J Case Rep Imag. 2012; 3:7–12.

10. Azuma K, Yashiro N, Kinoshita T, Yoshigi J, Ihara N. Hepatic involvement of visceral larva migrans due to Toxocara canis: a case report--CT and MR findings. Radiat Med. 2002; 20:89–92.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download