Abstract
Ankylosing spondylitis (AS) can involve the eye, gastrointestinal system, cardiopulmonary system, skin, kidneys, and spinal and peripheral joints. It is rarely accompanied by immunoglobulin A (IgA) nephropathy. Although IgA is involved in both AS and IgA nephropathy, the relationship between these diseases remains unclear. We detected hematuria and proteinuria in a 32-year-old male patient with ankylosing spondylitis that remained stable for 4 years through treatment with etanercept, a tumor necrosis factor-α (TNF-α) inhibitor, and diagnosed IgA nephropathy through a renal biopsy. IgA nephropathy seems to be less commonly associated with AS disease activity or specific treatment such as TNF-α inhibitor use.
Ankylosing spondylitis (AS), a type of spondyloarthritis, is a chronic inflammatory disorder that usually involves the spinal and peripheral joints. Uveitis is the most common extra-articular manifestation; other manifestations affect the gastrointestinal system, cardiopulmonary system, skin, and kidneys.1 Tumor necrosis factor-α (TNF-α) inhibitors are highly effective in the treatment of arthritis and uveitis in patients with AS, but their effects on the other clinical manifestations remain unclear.
Immunoglobulin A (IgA) nephropathy, the most common primary glomerular disease, is characterized by IgA infiltration in the mesangium and mesangioproliferative changes in the glomeruli. The etiopathogenesis of IgA nephropathy has not been thoroughly investigated.2 AS and IgA nephropathy share common immunological features, such as increased serum IgA and IgA immune complex levels, and a substantial portion of renal lesions associated with AS represent IgA nephropathy; thus, these diseases are considered to be closely related.3 However, as some studies have suggested that the prevalence rates of IgA nephropathy in healthy individuals and patients with AS do not vary significantly, the pathophysiological and epidemiological relationship is unclear.4
Here we report a case in which IgA nephropathy developed in a patient with AS whose overall disease activity was well controlled with etanercept, a TNF-α inhibitor.
A 32-year-old man visited the hospital with proteinuria and hematuria. The patient was diagnosed with AS at the same hospital 5 years prior. He was first administered meloxicam 15 mg/day and sulfasalazine 1,000 mg/day, but the response was insufficient. His regimen was changed to etanercept 50 mg/week and maintained for the past 4 years. The patient's Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score was relatively stable at 2.8–3.2 for several years with a relatively stable overall condition. No extra-articular manifestations of AS, such as uveitis, were reported. The patient had a history of asymptomatic renal stones. Intermittent microscopic hematuria was previously observed, but no additional testing was performed because it was considered indicative of asymptomatic renal stones. A regular medical examination conducted at the patient's workplace revealed microscopic hematuria and proteinuria, so he visited our hospital for a further evaluation. On a physical examination, his vital signs were stable with a blood pressure of 140/80 mmHg, pulse of 80/min, respiratory rate of 20/min, and body temperature of 37℃. There were no other abnormal findings.
A peripheral blood smear revealed a white blood cell count of 5,800/µl (neutrophils, 71%; lymphocytes, 7%), hemoglobin level of 14.5 g/dl, and platelet count of 217,000/mm3. The following serum biochemistry test results were obtained: total protein, 7.8 g/dl; albumin, 4.5 g/dl; total bilirubin, 0.7 mg/dl; aspartate transaminase, 34 IU/L; alanine transaminase, 36 IU/L; and lactate dehydrogenase, 333 IU/L. Blood urea nitrogen level was 14 mg/dL, creatinine level was 1.01 mg/dl, and estimated glomerular filtration rate (eGFR) was 92 mL/min. The erythrocyte sedimentation rate (ESR) was 10 mm/hr (normal, 0–16) and the high sensitivity C reactive protein (HS-CRP) level was 0.19 mg/dl (normal, 0.00–0.75). C3 level was 94.0 mg/dl (normal, 82–170), C4 level was 33.2 mg/dl (normal, 12–36), and CH50 level was 48.1 mg/dl (normal, 23–46). The patient was positive for HLA-B27, while negative for rheumatoid factor, antinuclear antibody, and antineutrophil cytoplasmic antibody. Furthermore, the patient was negative for hepatitis B antigen, hepatitis C antigen, and human immunodeficiency virus antigen, and was positive for hepatitis B antibody; venereal disease research laboratory tests yielded negative results. Serum protein electrophoresis (EP) findings were normal, and no particular findings were noted on immunofixation EP. In urinalysis, the microscopic white blood cell count was 1–4 cells/high power field (HPF), many red blood cells and proteins were observed, Bence-Jones protein was absent, urine protein electrophoresis was normal, and 24-hr urine protein level was 451 mg. The random urine protein/creatinine ratio was 287 mg/g. Mild bilateral sclerotic changes of the sacroiliac joint were observed on a pelvic radiograph (Fig. 1). On renal ultrasonography, the right kidney was 11.41 cm and the left kidney was 12.07 cm, both within normal range, and no abnormal findings were observed in the renal parenchyma. However, a 0.9-cm calculus was observed in the left kidney.
A light microscopic examination of renal biopsy specimens including 17 glomeruli revealed increased segmental deposition and cellularity of the mesangium. An immunofluorescence microscopic examination of a sample stained with antisera to IgG, IgA, IgM, C3, C4, and fibrinogen revealed negative findings for IgG, IgM, C3, C4, and fibrinogen but generalized granular deposition in the mesangium for IgA. Based on the above pathological findings, the patient was diagnosed with IgA nephropathy, Haas's histologic subclassification III (Fig. 2A, 2B). Inflammatory back pain was aggravated when the etanercept was discontinued for about 2 months after the renal biopsy, and since no clear causal relationship between IgA nephropathy and etanercept was identified, the drug was continued. For the proteinuria, ramipril 5 mg/day was used, but the patient developed a dry cough and the drug was changed to candesartan 8 mg/day. On a follow-up examination performed after 4 months, the microscopic hematuria was 5–10/HPF and the random urine protein/creatinine ratio was 185 mg/g. The patient remains in a stable condition without symptom exacerbation.
Although kidney involvement is uncommon in AS, it can be caused by concurrent conditions such as amyloidosis, nonsteroidal anti-inflammatory drug-induced nephropathy, or IgA nephropathy. In AS, secondary renal amyloidosis is the most common form of kidney involvement (62%), followed by IgA nephropathy (30%) and mesangioproliferative glomerulonephritis (5%). Membranous nephropathy (1%), focal segmental glomerulosclerosis (1%), focal proliferative glomerulonephritis (1%), and treatment-associated nephrotoxicity also occur in rare instances.5 In the present case, a patient with AS whose disease was well controlled with etanercept visited our hospital with incidentally detected proteinuria and hematuria and was confirmed to have concurrent IgA nephropathy on a kidney biopsy.
It is hypothesized that serum IgA immune complex in a patient with AS is the cause of IgA nephropathy or that certain genes such as HLA-B27 are involved in the concurrence of the two diseases, but some argue that there is neither a pathophysiological nor an epidemiological relationship between the two diseases.34 Among studies that emphasize the relationship between these two diseases, the concentration of serum IgA was related to the disease activity of AS and proteinuria in one report.6 Primarily, underglycated IgA1- and/or IgA-containing immune complexes are overproduced by B lymphocytes sensitized through the mucous membrane, and when they infiltrate the mesangium, the complement system is subsequently activated and mesangial growth factors such as platelet-derived growth factor are secreted. Glomerulonephritis, thought to be a consequence of this, progresses to renal tubulointerstitial scarring.78 The serum IgA concentration in patients with active AS may be increased, and the common elements of an increased IgA and/or IgA immune complex may cause concurrent nephropathy.3 In this case, although neither an IgA quantitative test nor a circulating immune complex test was performed, the relative distribution of IgA was found to be a normal fraction on serum protein EP and immune-fixation EP. Therefore, the role of IgA is assumed to be small.
TNF-α is known to induce glomerular inflammation and increase glomerular permeability.9 Thus, TNF-α inhibitors are assumed to theoretically reduce proteinuria in nephropathy; however, there is no strong basis for this belief. The reported cases of IgA nephropathy in patients with AS using TNF-α inhibitors are summarized in Table 1.11011 Unlike in the present case, monoclonal antibody agents such as infliximab or adalimumab were used in other cases. IgA nephropathy occurred when AS disease activity was stable in all cases. In this case, the results of disease activity evaluation, including BASDAI, ESR, and CRP, were within the normal ranges after etanercept treatment, and the patient's condition has remained stable for several years. This finding suggests that the occurrence of IgA nephropathy is less associated with different TNF-α inhibitors and AS disease activity. However, in this case, it is possible that the IgA nephropathy occurred because of the initially high AS disease activity before the use of etanercept and gradually progressed thereafter. Further studies on the possible association between AS disease activity and the occurrence of IgA nephropathy should be performed.
Meanwhile, the microscopic hematuria intermittently observed in this case was considered related to an asymptomatic calculus, while the possibility of renal involvement of AS (e.g., IgA nephropathy) was overlooked. Since various symptoms and comorbidities may accompany autoimmune rheumatic diseases, careful review and prompt action are recommended even in cases of relatively minor abnormal findings.
In this case, IgA nephropathy developed in a patient with AS that was well controlled with etanercept, a TNF-α inhibitor. The IgA nephropathy in this case was thought to have developed by mechanisms other than AS disease activity or specific treatment. Therefore, additional relevant studies should be conducted in the future.
Figures and Tables
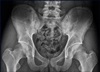 | Fig. 1Plain radiographs of the SI joint demonstrating grade 2 bilateral sacroiliitis. The film shows mild subchondral sclerosis and irregularities of the joint surfaces in both SI joints. |
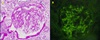 | Fig. 2(A) Light microscopy of a glomerulus demonstrating expansion of the mesangial matrix with mesangial hypercellularity (hematoxylin-eosin stain, ×400). (B) Immunofluorescence microscopy demonstrating anti-IgA intense staining within glomerular mesangium (fluorescence microscopy, ×400). |
Table 1
Studies of IgA nephropathy in patients with ankylosing spondylitis treated with various TNF inhibitors

ACEI, Angiotensin Converting Enzyme Inhibitor; ARB, Angiotensin Receptor Blocker; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; Cr, creatinine; eGFR, estimated glomerular filtration rate; HPF, high power field; ID, incorrect data; NR, not recorded; RBC, red blood cell; TNF, tumor necrosis factor;
References
1. Lee SH, Lee EJ, Chung SW, Song R, Moon JY, Lee SH, et al. Renal involvement in ankylosing spondylitis: prevalence, pathology, response to TNF-a blocker. Rheumatol Int. 2013; 33:1689–1692.

2. Chang JH, Kim DK, Kim HW, Park SY, Yoo TH, Kim BS, et al. Changing prevalence of glomerular diseases in Korean adults: a review of 20 years of experience. Nephrol Dial Transplant. 2009; 24:2406–2410.

3. Jennette JC, Ferguson AL, Moore MA, Freeman DG. IgA nephropathy associated with seronegative spondylarthropathies. Arthritis Rheum. 1982; 25:144–149.

4. van de Laar MA, Moens HJ, van der Korst JK. Absence of an association between ankylosing spondylitis and IgA nephropathy. Ann Rheum Dis. 1989; 48:262–264.

5. Strobel ES, Fritschka E. Renal diseases in ankylosing spondylitis: review of the literature illustrated by case reports. Clin Rheumatol. 1998; 17:524–530.

6. Franssen MJ, van de Putte LB, Gribnau FW. IgA serum levels and disease activity in ankylosing spondylitis: a prospective study. Ann Rheum Dis. 1985; 44:766–771.

7. Ihm CG. IgA nephropathy over 40 years. Korean J Med. 2009; 77:435–443.
8. Floege J, Moura IC, Daha MR. New insights into the pathogenesis of IgA nephropathy. Semin Immunopathol. 2014; 36:431–442.

9. McCarthy ET, Sharma R, Sharma M, Li JZ, Ge XL, Dileepan KN, et al. TNF-alpha increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J Am Soc Nephrol. 1998; 9:433.

10. Jacquet A, Francois H, Frangie C, Yahiaoui Y, Ferlicot S, Micelli C, et al. IgA nephropathy associated with ankylosing spondylitis is not controlled by infliximab therapy. Nephrol Dial Transplant. 2009; 24:3540–3542.

11. Ozçakar L, Ekiz T, Yalçın S, Akıncı A. IgA nephropathy in an ankylosing spondylitis patient during infliximab therapy: chicken, egg or mother and child reunion. Acta Reumatol Port. 2013; 38:310.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download