Abstract
Purpose
The present study investigated associations between dopamine transporter (DAT) availability and α-synuclein levels in cerebrospinal fluid, as well as synuclein gene (SNCA) transcripts, and the effect of single nucleotide polymorphism of SNCA on DAT availability in healthy subjects.
Materials and Methods
The study population comprised healthy controls who underwent 123I-FP-CIT single-photon emission computed tomography screening. Five SNCA probes were used to target the boundaries of exon 3 and exon 4 (SNCA-E3E4), transcripts with a long 3′UTR region (SNCA-3UTR-1, SNCA-3UTR-2), transcripts that skip exon 5 (SNCA-E4E6), and the rare short transcript isoforms that comprise exons 1–4 (SNCA-007).
Results
In total, 123 healthy subjects (male 75, female 48) were included in this study. DAT availability in the caudate nucleus (p=0.0661) and putamen (p=0.0739) tended to differ according to rs3910105 genotype. In post-hoc analysis, DAT availability in the putamen was lower in subjects of TT genotype than those of CC/CT (p=0.0317). DAT availability in the caudate nucleus also showed a trend similar to that in the putamen (p=0.0597). Subjects of CT genotype with rs3910105 showed negative correlations with DAT availability in the putamen with SNCA-E3E4 (p=0.037, rho=−0.277), and SNCA-E4E6 (p=0.042, rho=−0.270), but not those of CC/TT genotypes.
Parkinson's disease (PD) is the second most common progressive neurodegenerative disorder after Alzheimer's disease.1 It is characterized by the loss of dopaminergic neurons of the substantia nigra and the presence of intraneuronal cytoplasmic inclusions, known as Lewy bodies, which are aggregates of α-synuclein.1 To detect the loss of dopaminergic neurons in PD, dopamine transporter (DAT) imaging has been employed with single-photon emission computed tomography (SPECT) and positron emission tomography (PET).2 DAT is a sodium chloride-dependent transmembrane protein on the presynaptic dopaminergic nerve terminal, and controls dopamine levels by active reuptake from the synaptic cleft.345 DAT imaging allows quantifying of the dopaminergic system in the human brain6 and in vivo demonstration of striatal dopamine activity.5 As 123I-FP-CIT shows a higher affinity for striatal DAT density,3 it is one of the most widely used radiotracers in evaluating neurodegenerative disease.78
α-synuclein, a small, natively unfolded protein in presynaptic axon terminals91011 plays a role in regulation of dopamine biosynthesis and terminating dopamine neurotransmission by altering DAT-mediated uptake of synaptic dopamine.1213 Aggregates of α-synuclein protein are a neuropathological hall-mark of PD.1415 Many previous efforts have attempted to determine the usefulness of α-synuclein in cerebrospinal fluid (CSF), synuclein gene (SNCA), and single nucleotide polymorphisms (SNPs) as biomarkers for PD. Mean CSF α-synuclein levels are significantly lower in patients with typical PD, and may be a biomarker for PD diagnosis.16
SNCA is selectively expressed in blood cells and neurons.1017 Previous studies have evaluated alternative SNCA transcripts from blood as specific biomarkers for PD development. However, until now, the roles of SNCA transcripts are not well understood. Previous studies have reported a positive correlation between DAT imaging and SNCA transcripts in PD.1819 In the Parkinson's Progression Markers Initiative (PPMI), five SNCA transcripts, transcript with boundaries of exon 3 and exon 4 (SNCA-E3E4), transcripts with a long 3′UTR region (SNCA-3UTR-1, SNCA-3UTR-2), transcripts that skip exon 5 (SNCA-E4E6), and rare short transcript isoforms that comprise exons 1–4 (SNCA-007), were counted to evaluate their ability as specific biomarkers for PD. In genome-wide association study, several SNPs in SNCA were associated with PD.20 However, the effect of SNP in SNCA was not investigated.
In this regard, we hypothesized that α-synuclein levels in CSF, SNCA, and SNP of SNCA might have an effect on DAT availability in healthy subjects. Therefore, we investigated the association between DAT availability measured from 123I-FP-CIT SPECT and α-synuclein levels in CSF, as well as SNCA transcripts from venous blood, and the effect of SNP of SNCA on DAT availability in healthy subjects using the PPMI database.
Data used in the preparation of this article were obtained from the PPMI database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.21 The study population consisted of healthy controls who underwent 123IFP-CIT SPECT screening. According to PPMI criteria of healthy subjects, males or females with an age of 30 years or older at screening were included, and subjects with a neurological disorder, a first degree relative with idiopathic PD, Montreal Cognitive Assessment score of 26 or less, medications that might interfere with DAT SPECT scans, anticoagulants that might preclude safe completion of the lumbar puncture, or investigational drugs, and a condition that precludes the safe performance of routine lumbar puncture were excluded. Subjects without CSF α-synuclein, RNA synuclein, and genotyping were excluded. Medical history, CSF, RNA synuclein counts, and 123I-FP-CIT SPECT scans were downloaded. The PPMI study was approved by the local Institutional Review Boards of all participating sites (Institute for Neurodegenerative Disorders, University of Pennsylvania, University of California, Los Angeles, Coriell Institute for Medical Research, Clinical Trials Coordination Center, Laboratory of Neurogenetics; National Institute on Aging NIH, Institute for Neurodegenerative Disorders, Clinical Trials Statistical and Data Management Center, University of Iowa), and written informed consent was obtained from each subject at the time of enrollment for imaging data and clinical questionnaires. All methods were performed in accordance with the relevant guidelines and regulations.
123I-FP-CIT SPECT was performed during the screening visit for all subjects. SPECT scans were acquired 4±0.5 hrs after injection of 111–185 MBq of 123I-FP-CIT. Subjects were pretreated with iodine solution or perchlorate prior to injection to block thyroid uptake. Raw data were acquired into a 128×128 matrix stepping every 3 or 4 degrees for the total projections. Raw projection data were reconstructed using iterative ordered subset expectation maximization with HERMES (Hermes Medical Solutions, Stockholm, Sweden). The reconstructed images were transferred to pmod (PMOD Technologies LLC, Zürich, Switzerland) for subsequent processing, including attenuation correction.
Downloaded scans were loaded using pmod v3.6 (PMOD Technologies LLC) with a 123I-FP-CIT template.22 Specific binding of 123I-FP-CIT regarding DAT was calculated using a region of interest analysis. A standard set of volumes of interest (VOIs) defining the caudate nucleus and putamen based on the Automated Anatomical Labeling (AAL) atlas23 was defined. The cerebellum was chosen as a reference region. VOI template was applied to measure specific binding ratios (SBRs) of the caudate nucleus, putamen, and striatum as follows: SBR=(target-cerebellum)/cerebellum. As semi-automatic software (pmod v3.6, PMOD Technologies LLC) with predefined AAL atlas was used to analyze SPECT scans, inter- and intra-observer variation might be negligible.
α-synuclein in CSF was analyzed using an enzyme-linked immunosorbent assay (Covance Research Products Inc., Denver, PA, USA) according to the manufacturer's instructions. Samples were run in duplicate, and data used for this study met quality control measures for technical performance. Venous blood was collected from each subject in PAXgene tubes (Qiagen, Valencia, CA, USA), incubated at room temperature for 24 hours, and frozen and shipped on dry ice to Coriell. RNA was extracted following the PAXgene procedure. Probes for the target and control RNAs were multiplexed and assayed on the nCounter Digital Analyzer (NanoString Technologies, Inc.). Five SNCA probes were used to target the boundaries of exon 3 and exon 4 (SNCA-E3E4), transcripts with a long 3′UTR region (SNCA-3UTR-1, SNCA-3UTR-2), transcripts that skip exon 5 (SNCA-E4E6), and the rare short transcript isoforms that comprise exons 1–4 (SNCA-007). Transcript counts were assayed in human blood in a high-precision NanoString gene expression assay that directly measures RNA counts in total RNA without bias from reverse transcription into cDNA. No template controls containing water substituted for template were included. To control for plate-to-plate variation and drift, equal amounts of RNA derived from Human Universal Reference RNA (Agilent Technologies) were spotted at the beginning, end, and throughout the entire experiment.
All samples were genotyped using Illumina NeuroX array following the manufacturer's protocol (Illumina, Inc., San Diego, CA, USA). The Genotyping Analysis Module within Genome Studio version 1.9.4 was used to analyze data. The genotype frequencies for SNPs were in Hardy-Weinberg Equilibrium (>0.05), and minor allele frequency was more than 0.05.
Normality was examined using D'Agostino-Pearson omnibus test. Kruskal-Wallis test was used to compare three subgroups of SNPs. Post-hoc analysis was done to compare two subgroups of SNPs using Mann-Whitney test. Spearman correlation was used to investigate the relationship between DAT availability and synuclein. Statistical analyses were performed using GraphPad Prism 7 for Mac OS X (GraphPad Software Inc., San Diego, CA, USA), and MedCalc software package (ver. 12.6.0.0, MedCalc, Mariakerke, Belgium).
In total, 123 healthy subjects (male 75, female 48) were included in this study. rs3910105 was in Hardy-Weinberg Equilibrium, and the minor allele frequency was 0.47. According to genotyping of rs3910105, 30 subjects were of CC genotype, 57 of CT, and 36 of TT. rs3910105 did not show any effect on CSF α-synuclein (p=0.8026), and RNA synuclein (SNCA-007, p=0.1137; SNCA-3UTR-1, p=0.8894; SNCA-3UTR-2, p=0.7179; SNCA-E3E4, p=0.2826; SNCA-E4E6, p=0.4110) (Table 1). However, DAT availability in the caudate nucleus (p=0.0661) and putamen (p=0.0739) tended to differ according to rs3910105 genotype. In post-hoc analysis, DAT availability in the putamen was lower in subjects of TT genotype than those of CC/CT (p=0.0317). DAT availability in the caudate nucleus tended to be similar with that in the putamen (p=0.0597) (Fig. 1).
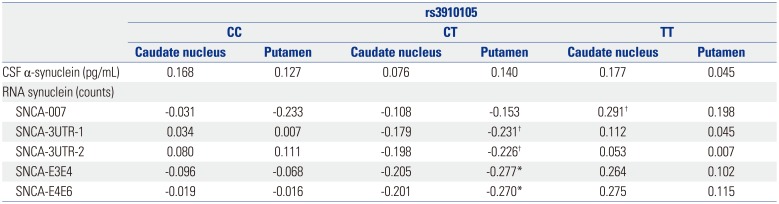
The correlation between DAT availability and synuclein was not significant in 123 subjects. However, after dividing subjects into 3 groups according to rs3910105, those with CT genotype of rs3910105 showed negative correlations of DAT availabilities of putamen with SNCA-E3E4 (p=0.037, rho=−0.277), and SNCA-E4E6 (p=0.042, rho=−0.270) (Fig. 2). In addition, SNCA-3UTR-1 (p=0.084, rho=−0.231), and SNCA-3UTR-2 (p=0.091, rho=−0.226) also showed the trend toward negative correlation with DAT availability of putamen in those with CT genotype (Table 2). However, in subjects with CC and TT genotypes did not show the meaningful association between DAT availability and synuclein.
In this study, rs3910105 appeared to effect DAT availability in the striatum. In subjects with CT genotype, higher DAT availability in the striatum and significant correlations with SNCA transcripts (SNCA-E3E4, SNCA-E4E6) were noted.
rs3910105, a SNP of SNCA, is a significant predictor of change in β-amyloid 1–42 (Aβ42), which has been shown to be relevant in PD neurodegeneration.24 However, rs3910105 shows a relatively subtle association with temporal Lewy body counts in the brain, and it seems to play a limited role in the degree to which Lewy body pathology progresses.25 Additionally, rs3910105 was not shown to be associated with cognitive decline in untreated PD patients.26 Still, the role of rs3910105 in PD, as well as healthy subjects, has remained unclear. In this study, SNCA transcripts showed a significant correlation with DAT availability in subjects of CT genotype, not in those of CC or TT. Until now, as this is the first study to report the effect of rs3910105 and a correlation between SNCA transcripts and DAT availability, the underlying mechanism of this association is not clear.
Previous studies have reported the effects of genotypes on DAT availabilities. CC genotypes with rs5443 of the G-protein β3 subunit gene showed higher DAT availability measured by 99mTc-TRODAT-1 SPECT than other genotypes.27 The effect of a variable number tandem repeat of SLC6A3 on DAT availability is well-known.2829 In addition, the effect of rs53576 of oxytocin receptor gene was also reported, and genetic variation in the common downstream signaling molecule of dopamine autoreceptors may affect the dysregulation of the striatal dopamine system.2730 As aggregations of α-synuclein are considered to be a central factor in the development of Lewy bodies in PD119 and as the expressions of SNCA transcripts in healthy subjects were higher than those in PD,31 we hypothesized that SNCA transcripts are correlated positively with DAT availability. However, contrary to our expectations, the correlation between SNCA transcripts and DAT availability was significant, although negatively in subjects with CT genotype of rs3910105. α-synuclein consists of three domains with a N-terminal lipidbinding α-helix, amyloid-binding central domain, and C-terminal acidic tail,3233 which has a function in suppression of apoptosis, regulation of glucose, modulation of calmodulin activity, chaperone activity, and regulation of dopamine biosynthesis.32 Although the role of the majority of SNCA transcripts remains unknown, SNCA-3UTR is associated with the accumulation of long transcripts;34 SNCA-E4E6 leads to shortening of α-synuclein C-terminal.17 In addition, reduced SNCA transcripts, especially SNCA-3UTR and SNCA-E4E6, might have a clinical role as potential predictors of cognitive decline in PD.31 In addition, Gao, et al.18 reported a positive correlation between SNCA transcripts and cerebral vesicular monoamine transporter 2 binding measured from 18F-AV133 PET in PD. However, 8 patients were included in this study, and PD patients were not categorized according to genotypes of SNP of SNCA.
This is the first study to report the effect of rs3910105 on DAT availability in the striatum and correlations between SNCA transcripts and DAT availability. However, there are several limitations in this study. Until now, as there are no previous studies regarding rs3910105 and SNCA transcripts, we could not explain the underlying mechanisms of this phenomenon. Secondly, although we included as many as 133 healthy subjects, we could not evaluate the reproducibility of our results using other databases. Further studies with a prospective design and basic research might be needed to clarify the association between SNCA transcripts and DAT availability.
In conclusion, this is the first study to investigate the association of rs3910105 of SNCA with DAT availability measured from 123I-FP-CIT SPECT. rs3910105 had an effect on DAT availability, and the correlation between DAT availability and SNCA transcripts were significant in CT genotypes of rs3910105.
ACKNOWLEDGEMENTS
PPMI-a public-private partnership-is funded by the Michael J. Fox Foundation for Parkinson's Research and funding partners, including abbVie, Avid, Biogen, Bristol-Myers Squibb, COVANCE, GE Healthcare, Genentech, GlaxoSmithKline, Lundbeck, Lilly, Merck, MesoScaleDiscovery, Pfizer, Piramal, Roche, Sanofi Genzyme, Servier, TEVA, and UCB.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) (2017R1D1A1B03029352/2017R1D1A1B03033235/2018R1C1B5030638). The funding source had no role in the collection of the data or in the decision to submit the manuscript for publication.
References
1. Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998; 95:6469–6473. PMID: 9600990.
2. Politis M. Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat Rev Neurol. 2014; 10:708–722. PMID: 25385334.

3. Park E. A new era of clinical dopamine transporter imaging using 123I-FP-CIT. J Nucl Med Technol. 2012; 40:222–228. PMID: 23160562.

4. Booth TC, Nathan M, Waldman AD, Quigley AM, Schapira AH, Buscombe J. The role of functional dopamine-transporter SPECT imaging in parkinsonian syndromes, part 1. AJNR Am J Neuroradiol. 2015; 36:229–235. PMID: 24904053.

5. Marshall V, Grosset D. Role of dopamine transporter imaging in routine clinical practice. Mov Disord. 2003; 18:1415–1423. PMID: 14673877.

6. Zipursky RB, Meyer JH, Verhoeff NP. PET and SPECT imaging in psychiatric disorders. Can J Psychiatry. 2007; 52:146–157. PMID: 17479522.

7. Thomas AJ, Attems J, Colloby SJ, O'Brien JT, McKeith I, Walker R, et al. Autopsy validation of 123I-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology. 2017; 88:276–283. PMID: 27940650.

8. Koch W, Unterrainer M, Xiong G, Bartenstein P, Diemling M, Varrone A, et al. Extrastriatal binding of [123I]FP-CIT in the thalamus and pons: gender and age dependencies assessed in a European multicentre database of healthy controls. Eur J Nucl Med Mol Imaging. 2014; 41:1938–1946. PMID: 24806112.
9. Stefanis L. α-Synuclein in Parkinson's disease. Cold Spring Harb Perspect Med. 2012; 2:a009399. PMID: 22355802.

10. Beyer K. Alpha-synuclein structure, posttranslational modification and alternative splicing as aggregation enhancers. Acta Neuropathol. 2006; 112:237–251. PMID: 16845533.
11. Bisaglia M, Mammi S, Bubacco L. Structural insights on physiological functions and pathological effects of alpha-synuclein. FASEB J. 2009; 23:329–340. PMID: 18948383.
12. Sidhu A, Wersinger C, Vernier P. alpha-Synuclein regulation of the dopaminergic transporter: a possible role in the pathogenesis of Parkinson's disease. FEBS Lett. 2004; 565:1–5. PMID: 15135042.
13. Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002; 22:3090–3099. PMID: 11943812.
14. Spillantini MG, Goedert M. The alpha-synucleinopathies: Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. Ann N Y Acad Sci. 2000; 920:16–27. PMID: 11193145.
15. Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rüb U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson's disease (preclinical and clinical stages). J Neurol. 2002; 249(Suppl 3):III/1–III/5.

16. Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Döring F, Trenkwalder C, Schlossmacher MG. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol. 2011; 10:230–240. PMID: 21317042.

17. Beyer K, Domingo-Sábat M, Lao JI, Carrato C, Ferrer I, Ariza A. Identification and characterization of a new alpha-synuclein isoform and its role in Lewy body diseases. Neurogenetics. 2008; 9:15–23. PMID: 17955272.

18. Gao R, Zhang G, Chen X, Yang A, Smith G, Wong DF, et al. CSF biomarkers and its associations with 18F-AV133 cerebral VMAT2 binding in Parkinson's disease-a preliminary report. PLoS One. 2016; 11:e0164762. PMID: 27764160.

19. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997; 388:839–840. PMID: 9278044.
20. Edwards TL, Scott WK, Almonte C, Burt A, Powell EH, Beecham GW, et al. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet. 2010; 74:97–109. PMID: 20070850.
21. Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011; 95:629–635. PMID: 21930184.
22. García-Gómez FJ, García-Solís D, Luis-Simón FJ, Marín-Oyaga VA, Carrillo F, Mir P, et al. [Elaboration of the SPM template for the standardization of SPECT images with 123I-Ioflupane]. Rev Esp Med Nucl Imagen Mol. 2013; 32:350–356. PMID: 23570700.

23. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002; 15:273–289. PMID: 11771995.

24. Mollenhauer B, Caspell-Garcia CJ, Coffey CS, Taylor P, Shaw LM, Trojanowski JQ, et al. Longitudinal CSF biomarkers in patients with early Parkinson disease and healthy controls. Neurology. 2017; 89:1959–1969. PMID: 29030452.

25. Heckman MG, Kasanuki K, Diehl NN, Koga S, Soto A, Murray ME, et al. Parkinson's disease susceptibility variants and severity of Lewy body pathology. Parkinsonism Relat Disord. 2017; 44:79–84. PMID: 28917824.

26. Caspell-Garcia C, Simuni T, Tosun-Turgut D, Wu IW, Zhang Y, Nalls M, et al. Multiple modality biomarker prediction of cognitive impairment in prospectively followed de novo Parkinson disease. PLoS One. 2017; 12:e0175674. PMID: 28520803.

27. Chen PS, Yeh TL, Lee IH, Lin CB, Tsai HC, Chen KC, et al. Effects of C825T polymorphism of the GNB3 gene on availability of dopamine transporter in healthy volunteers--a SPECT study. Neuroimage. 2011; 56:1526–1530. PMID: 21371559.
28. van de Giessen E, de Win MM, Tanck MW, van den Brink W, Baas F, Booij J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. J Nucl Med. 2009; 50:45–52. PMID: 19091889.

29. van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med. 2005; 46:745–751. PMID: 15872345.
30. Fazio L, Blasi G, Taurisano P, Papazacharias A, Romano R, Gelao B, et al. D2 receptor genotype and striatal dopamine signaling predict motor cortical activity and behavior in humans. Neuroimage. 2011; 54:2915–2921. PMID: 21087673.

31. Locascio JJ, Eberly S, Liao Z, Liu G, Hoesing AN, Duong K, et al. Association between α-synuclein blood transcripts and early, neuroimaging-supported Parkinson's disease. Brain. 2015; 138(Pt 9):2659–2671. PMID: 26220939.

32. Emamzadeh FN. Alpha-synuclein structure, functions, and interactions. J Res Med Sci. 2016; 21:29. PMID: 27904575.

33. Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017; 23:1–13.

34. Rhinn H, Qiang L, Yamashita T, Rhee D, Zolin A, Vanti W, et al. Alternative α-synuclein transcript usage as a convergent mechanism in Parkinson's disease pathology. Nat Commun. 2012; 3:1084. PMID: 23011138.

Fig. 1
Post-hoc analysis of dopamine transporter availability in the caudate nucleus (p=0.0597) and putamen (p=0.0317) according to genotypes of rs3910105.
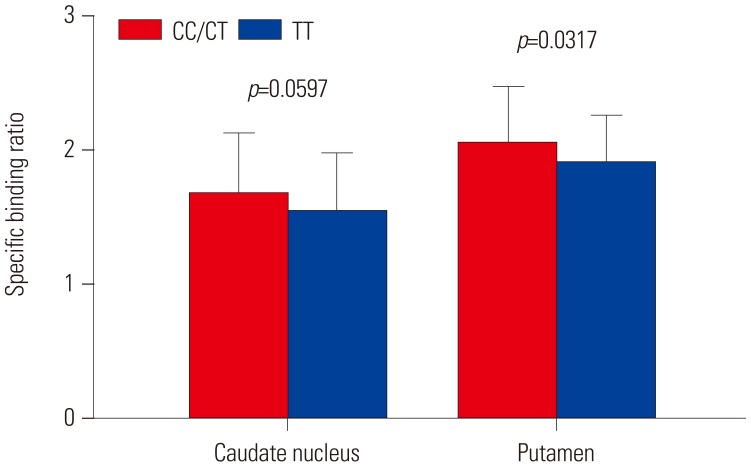
Fig. 2
Correlation between dopamine transporter availability of putamen in subjects with CT genotype of rs3910105 and (A) SNCA-E3E4 (p=0.037, rho=−0.277) and (B) SNCA-E4E6 (p=0.042, rho=−0.270).
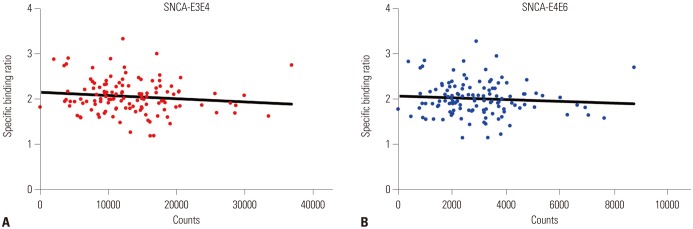
Table 1
Subject Characteristics according to Genotype of rs3910105
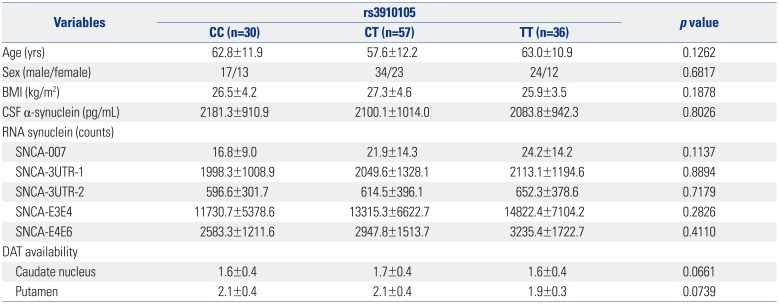
Table 2
Correlation Coefficient (rho) between Dopamine Transporter Availability and Synuclein in Subjects according to Genotype of rs3910105




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download