1. Woerner M, Sendtner E, Springorum R, Craiovan B, Worlicek M, Renkawitz T, et al. Visual intraoperative estimation of cup and stem position is not reliable in minimally invasive hip arthroplasty. Acta Orthop. 2016; 87:225–230. PMID:
26848628.

2. Renkawitz T, Haimerl M, Dohmen L, Gneiting S, Wegner M, Ehret N, et al. Minimally invasive computer-navigated total hip arthroplasty, following the concept of femur first and combined anteversion: design of a blinded randomized controlled trial. BMC Musculoskelet Disord. 2011; 12:192. PMID:
21854588.

3. Tsukeoka T, Tsuneizumi Y, Lee TH. A useful anatomical reference guide for stem anteversion during total hip arthroplasty in the dydsplastic hip. J Arthroplasty. 2015; 30:1393–1396. PMID:
25873282.
4. Tsukeoka T, Tsuneizumi Y, Lee TH. The T-line as an intraoperative landmark for reproducing the native femoral anteversion during hip arthroplasty. Arch Orthop Trauma Surg. 2014; 134:873–879. PMID:
24682493.

5. Hirata M, Nakashima Y, Ohishi M, Hamai S, Hara D, Iwamoto Y. Surgeon error in performing intraoperative estimation of stem anteversion in cementless total hip arthroplasty. J Arthroplasty. 2013; 28:1648–1653. PMID:
23602234.

6. Unlu MC, Kesmezacar H, Kantarci F, Unlu B, Botanlioglu H. Intraoperative estimation of femoral anteversion in cementless total hip arthroplasty using the lesser trochanter. Arch Orthop Trauma Surg. 2011; 131:1317–1323. PMID:
21359870.

7. Imai N, Ito T, Takahashi Y, Horigome Y, Suda K, Miyasaka D, et al. Do femoral neck and stem anteversion affect final femur rotation and pelvic positioning after total hip arthroplasty? Open J Orthopedics. 2013; 3:183–188.

8. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978; 60:217–220. PMID:
641088.

9. Lucas DH, Scott RD. The Ranawat sign. A specific maneuver to assess component positioning in total hip arthroplasty. J Orthop Tech. 1994; 2:59–62.
10. Bargar WL, Jamali AA, Nejad AH. Femoral anteversion in THA and its lack of correlation with native acetabular anteversion. Clin Orthop Relat Res. 2010; 468:527–532. PMID:
19714389.

11. Yun HH, Yoon JR, Yang JH, Song SY, Park SB, Lee JW. A validation study for estimation of femoral anteversion using the posterior lesser trochanter line: an analysis of computed tomography measurement. J Arthroplasty. 2013; 28:1776–1780. PMID:
23523486.
12. Zenk K, Finze S, Kluess D, Bader R, Malzahn J, Mittelmeier W. [Influence of surgeon experience in total hip arthroplasty. Dependence on operating time and complication risk]. Orthopade. 2014; 43:522–528. PMID:
24816976.
13. Dorr LD, Wan Z, Malik A, Zhu J, Dastane M, Deshmane P. A comparison of surgeon estimation and computed tomographic measurement of femoral component anteversion in cementless total hip arthroplasty. J Bone Joint Surg Am. 2009; 91:2598–2604. PMID:
19884433.

14. Wines AP, McNicol D. Computed tomography measurement of the accuracy of component version in total hip arthroplasty. J Arthroplasty. 2006; 21:696–701. PMID:
16877155.

15. Dorr LD, Malik A, Dastane M, Wan Z. Combined anteversion technique for total hip arthroplasty. Clin Orthop Relat Res. 2009; 467:119–127. PMID:
18979146.

16. Weber M, Woerner ML, Sendtner E, Völlner F, Grifka J, Renkawitz TF. Even the intraoperative knowledge of femoral stem anteversion cannot prevent impingement in total hip arthroplasty. J Arthroplasty. 2016; 31:2514–2519. PMID:
27236745.

17. Jerosch J, von Hasselbach C, Filler T, Peuker E, Rahgozar M, Lahmer A. [Increasing the quality of preoperative planning and intraoperative application of computer-assisted systems and surgical robots--an experimental study]. Chirurg. 1998; 69:973–976. PMID:
9816457.
18. Suh KT, Kang JH, Roh HL, Moon KP, Kim HJ. True femoral anteversion during primary total hip arthroplasty: use of postoperative computed tomography-based sections. J Arthroplasty. 2006; 21:599–605. PMID:
16781415.
19. Budin E, Chandler E. Measurement of femoral neck anteversion by a direct method. Radiology. 1957; 69:209–213. PMID:
13453733.
20. Lee YK, Kim TY, Ha YC, Kang BJ, Koo KH. Radiological measurement of femoral stem version using a modified Budin method. Bone Joint J. 2013; 95-B:877–880. PMID:
23814236.

21. Park J, Kim JY, Kim HD, Kim YC, Seo A, Je M, et al. Analysis of acetabular orientation and femoral anteversion using images of three-dimensional reconstructed bone models. Int J Comput Assist Radiol Surg. 2017; 12:855–864. PMID:
28063078.

22. Craiovan B, Renkawitz T, Weber M, Grifka J, Nolte L, Zheng G. Is the acetabular cup orientation after total hip arthroplasty on a two dimension or three dimension model accurate? Int Orthop. 2014; 38:2009–2015. PMID:
24737148.

23. Kitada M, Sakai T, Murase T, Hanada T, Nakamura N, Sugano N. Validation of the femoral component placement during hip resurfacing: a comparison between the conventional jig, patient-specific template, and CT-based navigation. Int J Med Robot. 2013; 9:223–229. PMID:
23460526.

24. Kunz M, Rudan JF, Xenoyannis GL, Ellis RE. Computer-assisted hip resurfacing using individualized drill templates. J Arthroplasty. 2010; 25:600–606. PMID:
19464848.

25. Raaijmaakers M, Gelaude F, De Smedt K, Clijmans T, Dille J, Mulier M. A custom-made guide-wire positioning device for hip surface replacement arthroplasty: description and first results. BMC Musculoskelet Disord. 2010; 11:161. PMID:
20630093.

26. Ito H, Tanaka S, Tanaka T, Oshima H, Tanaka S. A patient-specific instrument for femoral stem placement during total hip arthroplasty. Orthopedics. 2017; 40:e374–e377. PMID:
27841929.

27. Birkfellner W, Watzinger F, Wanschitz F, Ewers R, Bergmann H. Calibration of tracking systems in a surgical environment. IEEE Trans Med Imaging. 1998; 17:737–742. PMID:
9874297.

28. Matsumoto N, Hong J, Hashizume M, Komune S. A minimally invasive registration method using surface template-assisted marker positioning (STAMP) for image-guided otologic surgery. Otolaryngol Head Neck Surg. 2009; 140:96–102. PMID:
19130970.

29. Oka M, Cho B, Matsumoto N, Hong J, Jinnouchi M, Ouchida R, et al. A preregistered STAMP method for image-guided temporal bone surgery. Int J Comput Assist Radiol Surg. 2014; 9:119–126. PMID:
23801450.

30. Horn BK. Closed-form solution of absolute orientation using unit quaternions. J Opt Soc Am A. 1987; 4:629–642.

31. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1:307–310. PMID:
2868172.

32. Heller MO, Bergmann G, Deuretzbacher G, Claes L, Haas NP, Duda GN. Influence of femoral anteversion on proximal femoral loading: measurement and simulation in four patients. Clin Biomech (Bristol, Avon). 2001; 16:644–649.

33. Kleemann RU, Heller MO, Stoeckle U, Taylor WR, Duda GN. THA loading arising from increased femoral anteversion and offset may lead to critical cement stresses. J Orthop Res. 2003; 21:767–774. PMID:
12919861.

34. Kitada M, Nakamura N, Iwana D, Kakimoto A, Nishii T, Sugano N. Evaluation of the accuracy of computed tomography-based navigation for femoral stem orientation and leg length discrepancy. J Arthroplasty. 2011; 26:674–679. PMID:
20870379.

35. Imai H, Miyawaki J, Kamada T, Maruishi A, Takeba J, Miura H. Preoperative planning and operative techniques of the shorter tapered stem compared to the metaphyseal fit stem in cementless total hip arthroplasty. J Arthroplasty. 2017; 32:1192–1199. PMID:
27913129.

36. Sioen W, Simon JP, Labey L, Van Audekercke R. Posterior transosseous capsulotendinous repair in total hip arthroplasty : a cadaver study. J Bone Joint Surg Am. 2002; 84-A:1793–1798. PMID:
12377910.
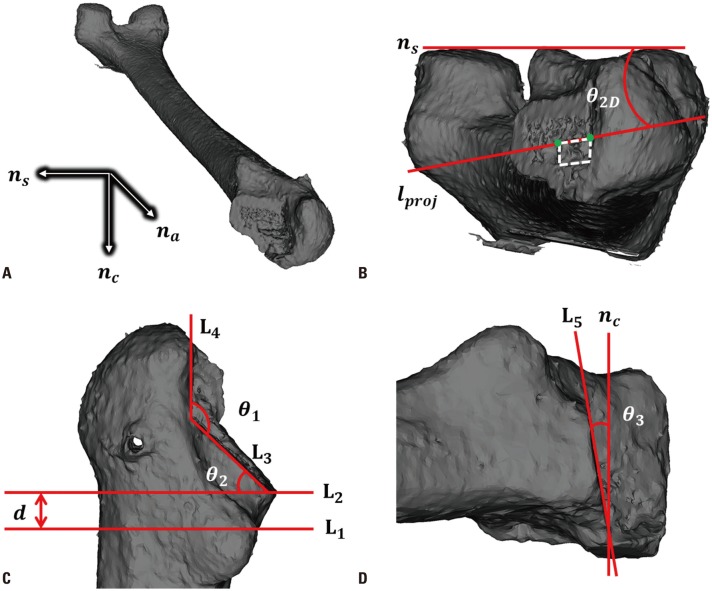
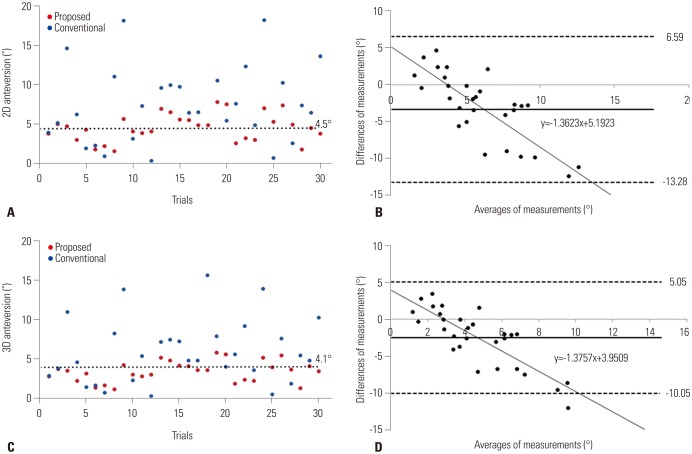
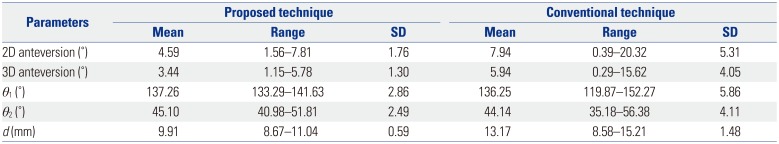
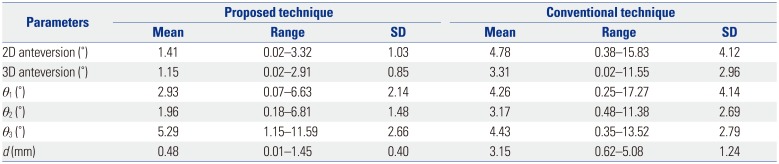




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download