|
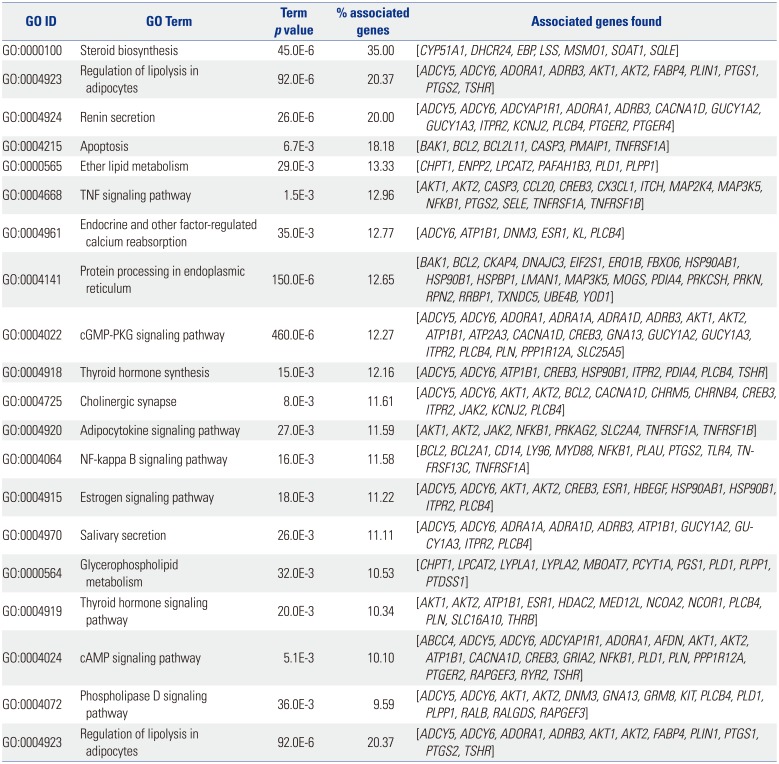
GO:0000100 |
Steroid biosynthesis |
4.50E-05 |
35.00 |
[CYP51A1, DHCR24, EBP, LSS, MSMO1, SOAT1, SQLE] |
|
GO:0004923 |
Regulation of lipolysis inadipocytes |
9.20E-05 |
20.37 |
[ADCY5, ADCY6, ADORA1, ADRB3, AKT1, AKT2, FABP4, PLIN1, PTGS1, PTGS2, TSHR] |
|
GO:0004924 |
Renin secretion |
2.60E-05 |
20.00 |
[ADCY5, ADCY6, ADCYAP1R1, ADORA1, ADRB3, CACNA1D, GUCY1A2, GUCY1A3, ITPR2, KCNJ2, PLCB4, PTGER2, PTGER4] |
|
GO:0004215 |
Apoptosis |
6.70E-03 |
18.18 |
[BAK1, BCL2, BCL2L11, CASP3, PMAIP1, TNFRSF1A] |
|
GO:0000565 |
Ether lipid metabolism |
2.90E-02 |
13.33 |
[CHPT1, ENPP2, LPCAT2, PAFAH1B3, PLD1, PLPP1] |
|
GO:0004668 |
TNF signaling pathway |
1.50E-03 |
12.96 |
[AKT1, AKT2, CASP3, CCL20, CREB3, CX3CL1, ITCH, MAP2K4, MAP3K5, NFKB1, PTGS2, SELE, TNFRSF1A, TNFRSF1B] |
|
GO:0004961 |
Endocrine and other factor-regulated calcium reabsorption |
3.50E-02 |
12.77 |
[ADCY6, ATP1B1, DNM3, ESR1, KL, PLCB4] |
|
GO:0004141 |
Protein processing in endoplasmic reticulum |
1.50E-04 |
12.65 |
[BAK1, BCL2, CKAP4, DNAJC3, EIF2S1, ERO1B, FBXO6, HSP90AB1, HSP90B1, HSPBP1, LMAN1, MAP3K5, MOGS, PDIA4, PRKCSH, PRKN, RPN2, RRBP1, TXNDC5, UBE4B, YOD1] |
|
GO:0004022 |
cGMP-PKG signaling pathway |
4.60E-04 |
12.27 |
[ADCY5, ADCY6, ADORA1, ADRA1A, ADRA1D, ADRB3, AKT1, AKT2, ATP1B1, ATP2A3, CACNA1D, CREB3, GNA13, GUCY1A2, GUCY1A3, ITPR2, PLCB4, PLN, PPP1R12A, SLC25A5] |
|
GO:0004918 |
Thyroid hormone synthesis |
1.50E-02 |
12.16 |
[ADCY5, ADCY6, ATP1B1, CREB3, HSP90B1, ITPR2, PDIA4, PLCB4, TSHR] |
|
GO:0004725 |
Cholinergic synapse |
8.00E-03 |
11.61 |
[ADCY5, ADCY6, AKT1, AKT2, BCL2, CACNA1D, CHRM5, CHRNB4, CREB3, ITPR2, JAK2, KCNJ2, PLCB4] |
|
GO:0004920 |
Adipocytokine signaling pathway |
2.70E-02 |
11.59 |
[AKT1, AKT2, JAK2, NFKB1, PRKAG2, SLC2A4, TNFRSF1A, TNFRSF1B] |
|
GO:0004064 |
NF-kappa B signaling pathway |
1.60E-02 |
11.58 |
[BCL2, BCL2A1, CD14, LY96, MYD88, NFKB1, PLAU, PTGS2, TLR4, TNFRSF13C, TNFRSF1A] |
|
GO:0004915 |
Estrogen signaling pathway |
1.80E-02 |
11.22 |
[ADCY5, ADCY6, AKT1, AKT2, CREB3, ESR1, HBEGF, HSP90AB1, HSP90B1, ITPR2, PLCB4] |
|
GO:0004970 |
Salivary secretion |
2.60E-02 |
11.11 |
[ADCY5, ADCY6, ADRA1A, ADRA1D, ADRB3, ATP1B1, GUCY1A2, GUCY1A3, ITPR2, PLCB4] |
|
GO:0000564 |
Glycerophospholipid metabolism |
3.20E-02 |
10.53 |
[CHPT1, LPCAT2, LYPLA1, LYPLA2, MBOAT7, PCYT1A, PGS1, PLD1, PLPP1, PTDSS1] |
|
GO:0004919 |
Thyroid hormone signaling pathway |
2.00E-02 |
10.34 |
[AKT1, AKT2, ATP1B1, ESR1, HDAC2, MED12L, NCOA2, NCOR1, PLCB4, PLN, SLC16A10, THRB] |
|
GO:0004024 |
cAMP signaling pathway |
5.10E-03 |
10.10 |
[ABCC4, ADCY5, ADCY6, ADCYAP1R1, ADORA1, AFDN, AKT1, AKT2, ATP1B1, CACNA1D, CREB3, GRIA2, NFKB1, PLD1, PLN, PPP1R12A, PTGER2, RAPGEF3, RYR2, TSHR] |
|
GO:0004072 |
Phospholipase D signaling pathway |
4.E-02 |
9.59 |
[ADCY5, ADCY6, AKT1, AKT2, DNM3, GNA13, GRM8, KIT, PLCB4, PLD1, PLPP1, RALB, RALGDS, RAPGEF3] |
|
GO:0004923 |
Regulation of lipolysis in adipocytes |
9.20E-05 |
20.37 |
[ADCY5, ADCY6, ADORA1, ADRB3, AKT1, AKT2, FABP4, PLIN1, PTGS1, PTGS2, TSHR] |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download