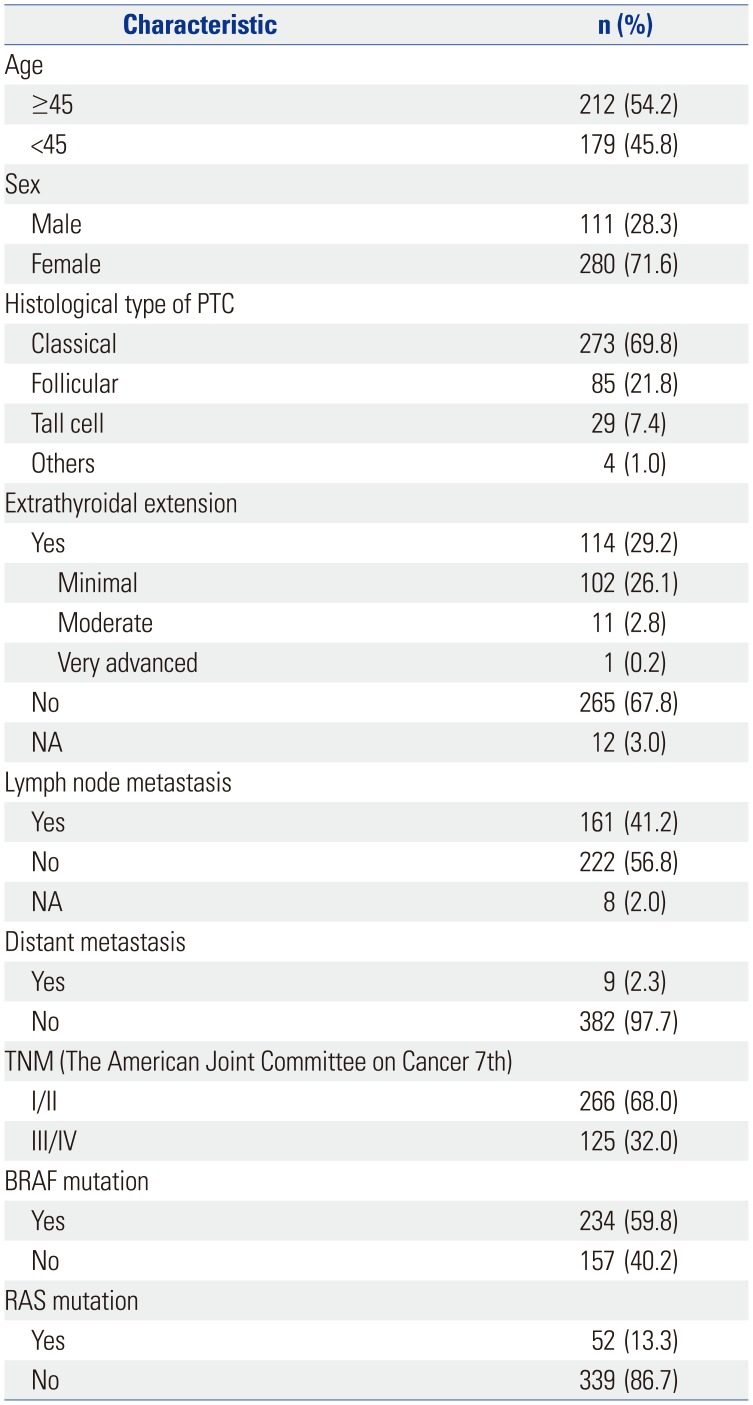
INTRODUCTION
The incidence of thyroid cancer continues to increase worldwide, including Korea.
12 Papillary thyroid cancer (PTC) usually has a favorable prognosis with an excellent survival rate.
12 However, a minority of PTC patients develop locoregional recurrence, including cervical lymph node metastases, and some of them may eventually succumb to the cancer.
3 Treatment of PTC employs a three-tiered approach, including surgery, radioactive iodine ablation (RAI), and long-term exogenous hormone replacement.
3 Following surgery and RAI, neck ultrasonography and whole-body radioactive iodine scan (WBS) are usually employed to detect residual tumors or metastasis.
3 Nowadays, positron emission tomography (PET) using
18F-fluorodeoxyglucose (FDG) has become a standard modality for staging, restaging, and monitoring treatment responses in a variety of tumors.
4 However, routine use of PET scans as a work-up is not recommended in thyroid cancer. In PTC patients with high thyroglobulin levels and negative WBS,
18F-FDG PET should be considered in order to detect suspected tumor recurrence or metastasis.
3
In the field of oncology, messenger RNA (mRNA) has emerged as a major molecular player in cancer genes, because mRNA is a core part of protein synthesis.
5 In cancer, mutated DNA is transcribed into complementary mRNA, which is then translated into malfunctioning cancer protein. However, few studies have investigated the role of mRNA as a prognostic predictor.
5 Recently, The Cancer Genome Atlas (TCGA) Research Network Initiative provided a detailed description of the genomic landscape of 509 PTC cases, named the “Integrated Genomic Characterization of PTC.”
6
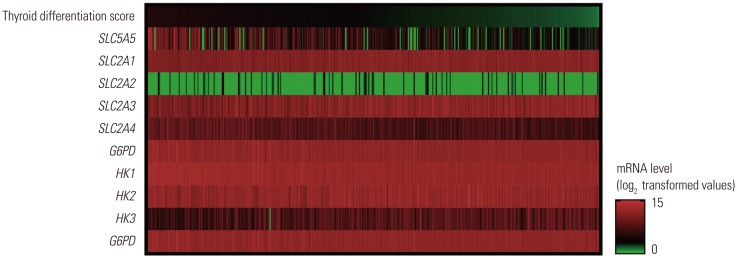
18F-FDG, a glucose analog, is transported to tumor cells via glucose transporter (GLUT) proteins and then phosphorylated by hexokinase (HK).
7
123I or
131I are radiopharmaceuticals that are applied in WBS and transported transcellularly to thyrocytes by the sodium/iodide symporter (NIS).
8 Therefore, we investigated mRNA expression of these genes, which are connected with FDG PET and WBS, to discern potential prognostic roles of mRNA using PTC data from TCGA.
Go to :

DISCUSSION
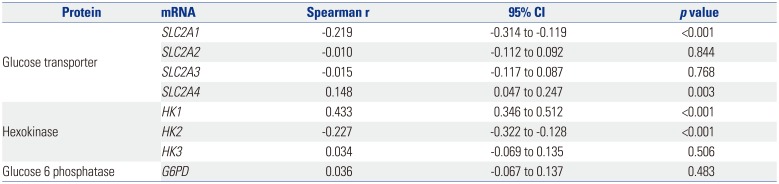
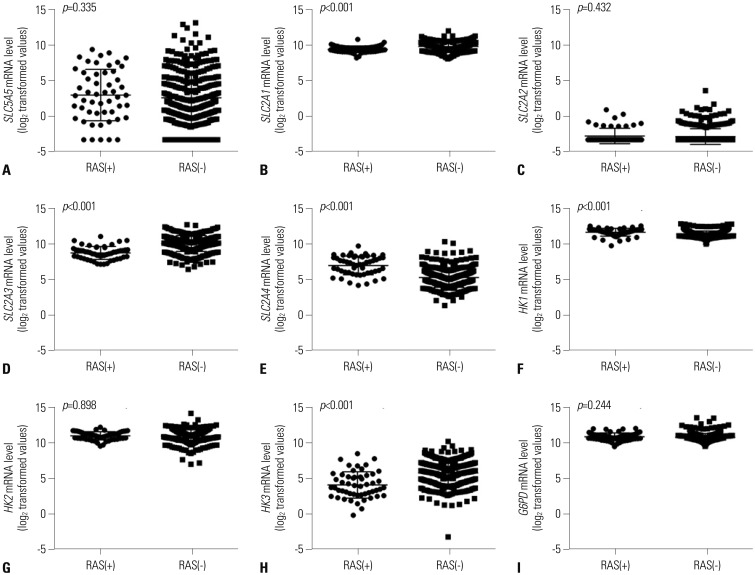
In this study, expression of
SLC5A5 mRNA was negatively correlated with that of
SLC2A1 and
HK2 and was positively correlated with
SLC2A4 and
HK1. During the natural evolution of differentiated thyroid cancer (DTC), either before diagnosis or over the course of treatment and follow-up, there is some loss of the ability to take up iodine owing to diminished
SLC5A5 expression. A defective iodide-trapping mechanism due to reduced
SLC5A5 expression appears to be an early and consistent feature of the oncogenic transformation of thyroid cells, and is associated with neoplastic transformation.
10 This phenomenon is often seen as DTC progresses to the later stages of cancer development, and explains why advanced, high-risk DTC patients have a poorer response to RAI than early-stage DTC patients.
11
Among the 14
SLC2A subtypes,
SLC2A1 has been most widely investigated in various cancer types, including thyroid cancer, wherein some studies have found increased
SLC2A1 expression.
1213 Furthermore, adverse effects of
SLC2A overexpression on survival outcomes in PTC patients have been implied for associations between
SLC2A1 overexpression and tumor aggressiveness or dedifferentiation.
1415 The expression of
SLC5A5 and
SLC2A1 mRNA is related to the accumulation of radioactive iodine (
123I or
131I) and
18F-FDG in thyroid cancer cells,
16 and the expression of these genes provides a molecular basis for the image modalities of gamma cameras and PET with these radiopharmaceuticals. High glucose uptake measured by FDG PET is associated with low
SLC5A5 expression, which indicates dedifferentiation in thyroid cancer.
17 This finding is a known phenomenon called “flip-flop” in FDG PET and WBS, which explains the mechanism of how FDG PET detects recurrent or metastatic cancer in patients, but WBS fails to detect tumors,
18 consistent with guidelines from the American Thyroid Association.
3 As a whole, our findings suggest that
SLC2A1 and
HK2 expression may be related to BRAF mutation and
18F-FDG uptake of PTC, which leads to cancer aggressiveness or dedifferentiation. Further studies are needed to clarify the relationship among
18F-FDG uptake, the expression of variable glucose transporters,
HK2, and BRAF mutations.
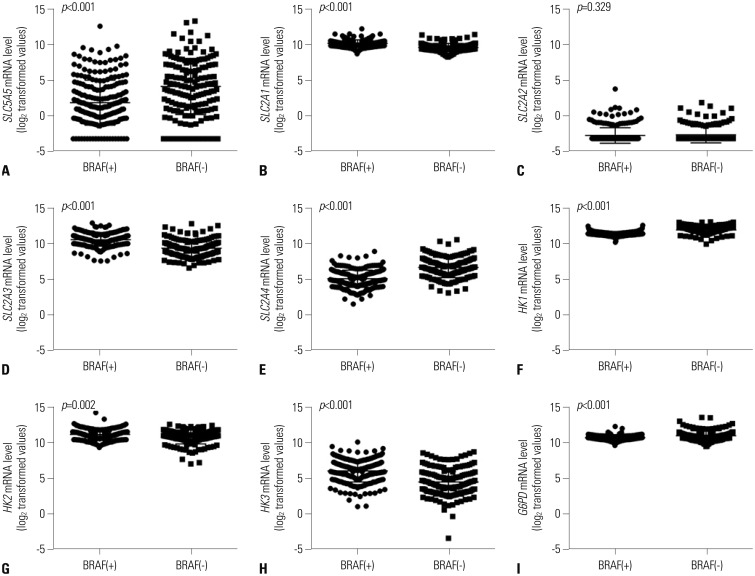
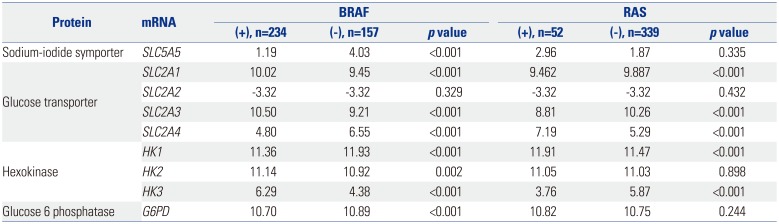
Most mRNA expression patterns in this study were significantly associated with PTC genetic mutations. Findings from a previous PTC TCGA showed that BRAF and RAS-like classes of PTC are significantly distinct in regards to differentiation state, and BRAF-like tumors exhibit a gene expression profile associated with a less differentiated state.
9 Morari, et al.
19 found that 230 DTC patients with a BRAF mutation had reduced
SLC5A5 mRNA abundance. In this study, expressions of
SLC2A1,
SLC2A3,
HK2, and
HK3 mRNA were higher in PTC patients with BRAF mutations, which might reflect excessive glucose influx required for cancer cell proliferation facilitated by
SLC2A,
20 a well-known prognostic marker of DTC.
21 This study is the first to show an association between mRNA and genetic mutation-prognosis. Our data suggest that mRNA expression helps to characterize a patient's risk and to identify individuals with a poor response to therapy. A larger number of patients with long-term follow-up may also confirm the clinical use of mRNA expression, reinforcing the predictive value of clinicopathological outcomes/markers, such as age and tumor size, as well as molecular markers, such as BRAF mutations.
We demonstrated that the expression of
SLC5,
SLC2, and
HK genes (associated with survival) was associated with BRAF and RAS mutations, a well-known, adverse prognostic factor in PTC.
21 The BRAF gene encodes a serine/threonine kinase that belongs to the RAS-RAF-MEK-ERK-MAP kinase pathway, whose biological role is to mediate cellular responses to growth factors.
12 Furthermore, we demonstrated that expression of
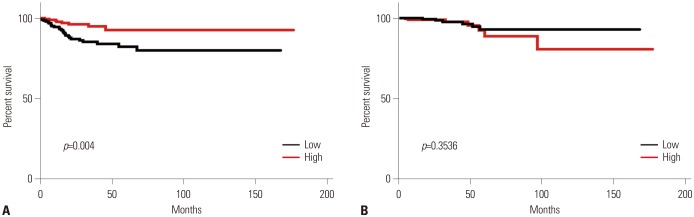
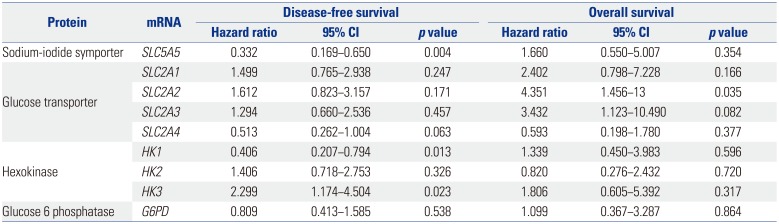
SLC5A5,
HK1, and
HK3 mRNA was associated with PTC recurrence. Extranodal extension is also associated with poor prognosis in thyroid cancer.
22 Our study is the first to show an association between mRNA expression and genetic mutation in cancer prognosis. Our data suggests that mRNA expression may help to determine patient risk and individuals with a poor response to therapy. Ward, et al.
23 identified that patients with PTC who experienced early recurrence or metastasis show low expression levels of
SLC5A5 mRNA in primary tumors. As expected,
SLC2A2 and
SLC2A3 mRNA, which are known to be associated with BRAF and RAS mutations, had unfavorable prognosis regarding OS. As lower mRNA expression of
SLC5A5 reflects a poorer response to radioactive iodine therapy, it is reasonable that this population of patients have favorable prognosis in DFS. However, mRNA expression of
SLC5A5 did not affect OS, as PTC is perceived to have a high survival rate, with a minority of patients showing recurrence or metastasis.
24 A larger number of patients with long-term follow-up may also confirm the clinical use of mRNA expression, reinforcing the predictive value of well-recognized clinical and pathological predictors of outcome.
Despite a vast amount of genetic and epigenetic information, the absence of solid prospective studies on thyroid cancer and information on the relationships among clinical, pathological, and genetic factors makes it difficult to discern the prognostic role played by genetic mutations.
5 The molecular characterization of thyroid cancer has begun to influence its diagnosis and treatment landscape. Gene expression analysis might also be clinically useful in the near future. In this study, we found evidence for the genetic basis of
18F-FDG PET in PTC patients with negative WBS with RAI and the prognostic value of
SLC5A5 in PTC. Additionally, there is a demand for new drugs for PTC refractory to standard treatment with RAI, as the impact of tyrosine kinase inhibitors on survival is not satisfactory and their side effects are often fatal.
12 In addition to prognosis, the aforementioned mutations and respective molecular pathways, as well as genetic and epigenetic alterations recently identified by TCGA,
9 may serve as likely targets and areas of focus for personalized therapy. Furthermore, drugs inhibiting
SLC2A-family genes might have great therapeutic potential by inducing starvation of tumor cells.
25 In addition,
SLC5A5 expression can be augmented by upregulation of both transcriptional and post-translational pathways. Some isoform-specific signal transduction pathways may play critical roles in tissue-specific
SLC5A5 regulation. Delineation of such signaling pathways may lead to methods that will further enhance functional
SLC5A5 expression in DTC, thus expanding the application of RAI to RAI-refractory PTC.
26 We could not analyze the other important genes related to iodine metabolism due to lack of data. In addition, we could only analyze expressions of mRNAs, not those of proteins. Further studies are needed to investigate the expression levels of proteins between
SLC2A family and
SLC5A5.
In conclusion, expression of SLC5A5 mRNA was negatively correlated with SLC2A1. This finding provides a molecular basis for the management of PTC patients with negative WBS using 18F-FDG PET scans. In addition, higher SLC5A5 mRNA expression was associated with less frequent recurrence or metastasis, but not with deaths. Moreover, PTC patients with BRAF mutations had reduced SLC5A5 mRNA expression and higher expression of HK2 and HK3 mRNA, which might reflect a less-differentiated state of PTC.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download