Abstract
Idiopathic hypereosinophilic syndrome (IHES) is a rare disease that is characterized by otherwise unexplained persistent eosinophilia and organ damage caused by eosinophilic infiltration. Its manifestations are highly variable but clinically apparent arthritis is uncommonly observed. Although Korean cases of severe eosinophilia in patients with rheumatoid arthritis (RA) or IHES concurrent with RA have been published, there are no reports of IHES with joint involvement. This paper reports a case of IHES presenting with persistent peripheral eosinophilia, fever, skin rash, multiple lymphadenopathy, and polyarthritis, including the distal interphalangeal joints of the hands.
Hypereosinophilic syndrome (HES) is a rare disorder characterized by peripheral eosinophilia (>1,500/mm3 on at least two occasions or for ≥6 months) and end-organ damage secondary to the eosinophilia [1]. The clinical features of HES are dependent on the organ system involved with tissue eosinophilia and HES commonly affects hematologic, cutaneous, cardiovascular, pulmonary, and neurologic systems. Concerning musculoskeletal involvement, myalgia and arthralgia can occur with HES [12]. Moreover, idiopathic HES (IHES) cases with joint involvement have been rarely described in English-written literature [3456]. However, similar clinical features have been also reported when HES developed in patients with long-standing or concurrent rheumatoid arthritis (RA) [789].
Although Korean cases of severe eosinophilia in patients with RA or IHES concurrent with RA have been published [8910], IHES with joint involvement has not been reported yet. Herein, we report a case of IHES with polyarthritis involving the distal interphalangeal (DIP) joints of the hands. The study was approved by the Institutional Review Board of the Seoul National University Bundang Hospital (IRB no. B-1801-447-701).
A 48-year old woman was admitted to Seoul National University Bundang Hospital for evaluation of recurrent febrile episodes, pruritic exfoliative skin rash, and polyarthralgia. She had been healthy until 9 months ago when she was diagnosed with adhesive capsulitis in both shoulders. After 1 month, she developed pain and swelling in both 2nd and the left 4th hand DIP joints and noted pruritic erythematous lesions on the trunk. Laboratory examinations at another hospital showed an increased eosinophil counts (843/mm3) and raised erythrocyte sedimentation rate (ESR, 98 mm/hr). Triamcinolone intra-articular injections relieved her symptoms for 1 month but pain and swelling recurred in the left 2nd DIP joints and eosinophil count remained persistently elevated (1,514/mm3). In addition, her erythematous papules extended to the upper and lower extremities and were accompanied with exfoliative scaling (Figure 1A), even with topical corticosteroid treatment. Five months ago, she was admitted to the hospital for treatment of weight loss (3 kg over 2 months) and anorexia, and had persistent eosinophilia (2,614/mm3). During the admission, she developed a febrile episode and received prednisolone (PD) 30 mg/day with a rapid tapering over 10 days. Nevertheless, her symptoms wax- and-waned and fever recurred with chilling sensation. The patient denied histories of recurrent oro-genital ulceration, photosensitivity, Raynaud's phenomenon, uveitis, and respiratory, gastrointestinal, or genitourinary symptoms although she had anorexia.
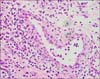
On admission, her body temperature was 37.9℃ and her skin exhibited generalized erythroderma and scaling (Figure 1B). Joint examination revealed tenderness and swelling at both wrist, the left 1st interphalangeal (IP), right 3rd proximal interphalangeal (PIP), both 2nd and 5th DIP, and left 4th DIP joints (Figure 2A). The impingement sign was positive at both shoulders along with tenderness over the supraspinatus. Laboratory tests showed the following: white blood cell count, 9,600/mm3; hemoglobin, 8.0 g/dL; eosinophil, 3,322/mm3; ESR, 95 mm/hr; C-reactive protein, 9.29 mg/dL; lactate dehydrogenase, 878 IU/mL (reference range, 100~225); eosinophil cationic protein, 47.6 ug/L (reference range, 2~18), and a negative result for rheumatoid factor (RF), anti-cyclic citrullinated peptide (CCP), and antinuclear antibody. Serum protein electrophoresis and immunoelectrophoresis were also normal. The stool examination for parasites or eggs was negative and parasite-specific antibodies against Clonorchis sinensis, Paragonimus westermani, Cysticercus, and Sparganum were all negative. Computed tomography (CT) showed splenomegaly and multiple lymphadenopathies in the neck, chest and abdominal cavity. The enlarged lymph nodes were hypermetabolic in the positron emission tomography/CT. Joint X-ray showed soft tissue swelling in PIP and DIP joints and joint space narrowing was observed in both 2nd DIP and the left wrist joints (Figure 2B). Skin biopsy showed a spongiotic dermatitis with perivascular lymphocytic infiltration with eosinophils. The inguinal lymph node and bone marrow biopsy revealed eosinophilic infiltration. There was no evidence of major gene deletion or rearrangement for lymphoproliferative disorders including fibroblast growth factor receptor-1 and platelet-derived growth factor receptor beta gene rearrangement. Synovial tissue was obtained from the right 3rd PIP joint and histopathology examination also showed chronic active inflammation with eosinophilic infiltration (Figure 3).
She was finally diagnosed with IHES involving skin, lymph node, bone marrow and peripheral joints including the hand DIP joints. Following the initiation of PD 20 mg/day (about 0.5 mg/kg/day) and cyclosporine 100 mg/day, her fever and arthralgia disappeared rapidly over 1 to 2 days, and the blood eosinophil count was normalized over 5 days. The patient was followed up with gradual tapering of the PD to 10 mg every other day. The recurrence of symptom or the development of radiographic progression was not observed for 6 months.
The current case was diagnosed as having IHES based on persistent eosinophilia (>1,500/mm3 for >6 months) without an identifiable etiology and eosinophilic infiltration in the target organs [1]. Clinical manifestations of HES depend on the organ system involved, and the skin, heart, lungs, and central or peripheral nervous system are involved in >50% of patients. Other clinical features include fever, hepatosplenomegaly, gastroenteritis, and coagulation disorder [12]. Therefore, fever, chronic pruritic skin rash with eosinophilic infiltration, and multiple eosinophilic lymphadenopathy in this case were considered attributed to the IHES.
In patients presenting with arthritis/arthralgia and elevated eosinophil counts, the differential diagnosis includes a variety of conditions including RA with eosinophilia, eosinophilic granulomatosis with polyangiitis, and eosinophilic arthritis [11]. Eosinophilia accompanying RA is sometimes found [10] and a recent study reported that eosinophilia in early RA could be a marker of higher disease activity and poor therapeutic response [12]. Another possibility would include RA concurrent with HES. Choi et al. [8] reported a case of seronegative RA concomitant with IHES and Park et al. [9] described a case of IHES preceding seropositive RA. However, joint manifestation in our case was not compatible with that of typical RA because of the negative RF and anti-CCP, eosinophilic synovial infiltration, and multiple DIP joint involvement. Additionally, our patient had no pathological evidence of vasculitis and her clinical features were not compatible with eosinophilic arthritis. Eosinophilic arthritis shows a lack of systemic symptoms and normal laboratory findings except for eosinophilia and many patients with eosinophilic arthritis respond to antiparasitic drugs [11].
Even though joint involvement is not common in patients with HES, arthritis/arthralgia has been described in previous literatures [124]. Through a search of PubMed and Google Scholar, we identified 3 English-written reports describing the joint involvement of HES patients in detail [356]. It took 2~8 years before establishing the diagnosis of IHES. All the cases showed a bilateral involvement of the wrist or hand joints and a predominant involvement of the upper extremities, despite negative RF. Interestingly, subcutaneous nodules over the joints were observed in 3 cases, and on pathological examination, the nodules showed eosinophilic infiltration. Joint X-rays exhibited no erosion or bony abnormality in 2 cases but one had destructive lesions in the metacarpophalngeal joint and 1st IP joints [6]. Therefore, some of their clinical features overlapped with those of RA. In this context, the case of reported by Choi et al. [8] might have articular involvement contributed by IHES not by seronegative RA; her synovial pathology revealed eosinophilic infiltration that is not a feature of RA synovitis. Our patients had a unique involvement of the hand DIP joint, but other articular manifestations were similar to those described in previous reports. Based on this, we finally concluded that her polyarthritis was secondary to IHES.
Generally, Th2 cytokines are considered anti-inflammatory in nature, and an eosinophil response could suppress inflammatory arthritis in experimental arthritis models [13]. It has often been reported that articular manifestations in HES include arthralgia or non-erosive arthritis [124]. However, cases by Anders and Schattenkirchner [6] as well as ours cases revealed radiographic joint damage. Our patients showed mild joint-space narrowing, but the case of Anders and Schattenkirchner [6] revealed more destructive change; it might be a result of the duration of arthritis (8 months versus 2 years). Additionally, eosinophils can produce a variety of proinflammatory mediators including non-canonical factors of osteoclast differentiation and matrix-degrading metalloproteinases [14]. Therefore, although joint damage is not a main feature in patients with HES, it could induce joint inflammation and tissue destruction.
Concerning the treatment of HES, corticosteroids have been one of the most widely utilized agents and second-line agents include hydroxyurea, interferon-α, tyrosine kinase inhibitors, and monoclonal antibodies to interleukin-5 [1]. The response to corticosteroids is known to be dramatic and rapid in most patients but about 75% require subsequent chronic maintenance therapy with corticosteroids. Because vital organs were not affected in the current case, we started a moderate dose of PD (0.5 mg/kg/day) and added cyclosporine as a steroid-sparing agent. Because eosinophilic granulopoiesis is thought to be under the control of T lymphocytes in HES, cyclosporine could be a reasonable therapeutic option. In addition, cyclosporine is the most commonly used anti-rheumatic drug for the treatment of IHES [15] and was reported to be successfully used in a patient with IHES and RA [7].
This is the first Korean case of IHES affecting the hand and wrist joints including the DIP joints. Our literature review shows that HES uncommonly affects the joints and can result in significant joint tissue damage in some patients. Because its articular manifestations could resemble those of RA, a synovial biopsy may be useful for a differential diagnosis between RA and HES-associated arthritis.
Figures and Tables
 | Figure 1Photographs of skin lesions. Erythematous and exfoliative eruptions (A) progressed into generalized erythroderma and scaling (B) over about 6 months. |
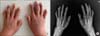 | Figure 2Articular manifestation. Photography (A) and X-ray (B, oblique) of both hands showed prominent swelling in the right 3rd proximal interphalangeal, left 1st interphalangeal, and both 2nd, left 4th, and both 5th distal interphalangeal (DIP) joints. Radiographic joint-space narrowing was observed in the left radio-lunar and radio-scaphoid joints and both 2nd DIP joints. |
References
3. Brogadir SP, Goldwein MI, Schumacher HR. A hypereosinophilic syndrome mimicking rheumatoid arthritis. Am J Med. 1980; 69:799–802.

4. Spry CJ, Davies J, Tai PC, Olsen EG, Oakley CM, Goodwin JF. Clinical features of fifteen patients with the hypereosinophilic syndrome. Q J Med. 1983; 52:1–22.
5. Martín-Santos JM, Mulero J, Andréu JL, de Villa LF, Bernaldo-de Quirós L, Noguera E. Arthritis in idiopathic hypereosinophilic syndrome. Arthritis Rheum. 1988; 31:120–125.
6. Anders HJ, Schattenkirchner M. Destructive joint lesions and bursitis in idiopathic hypereosinophilic syndrome. Rheumatology (Oxford). 1999; 38:185–186.

7. Chaudhuri K, Dubey S, Zaphiropoulos G. Idiopathic hypereosinophilic syndrome in a patient with long-standing rheumatoid arthritis: a case report. Rheumatology (Oxford). 2002; 41:349–350.

8. Choi JH, Jung JW, Song HJ, Song KE, Choi JH, Suh YJ, et al. A case of seronegative rheumatoid arthritis with idiopathic hypereosinophilic syndrome. J Korean Rheum Assoc. 2003; 10:200–205.
9. Park JH, Lee WS, Park SJ, Yoo WH. Hypereosinophilic syndrome associated with the onset of rheumatoid arthritis: a case report. J Rheum Dis. 2017; 24:165–168.

10. Sohn CI, Kim MK, Lee KC, Jung SS, Lee IH, Bae SC, et al. A case of rheumatoid arthritis accompanied by severe eosinophilia. J Korean Rheum Assoc. 1994; 1:98–102.
12. Guellec D, Milin M, Cornec D, Tobon GJ, Marhadour T, Jousse-Joulin S, et al. Eosinophilia predicts poor clinical outcomes in recent-onset arthritis: results from the ESPOIR cohort. RMD Open. 2015; 1:e000070.

13. Chen Z, Andreev D, Oeser K, Krljanac B, Hueber A, Kleyer A, et al. Th2 and eosinophil responses suppress inflammatory arthritis. Nat Commun. 2016; 7:11596.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download