Abstract
Inflammatory bowel disease (IBD) is a well-recognized risk factor for thrombotic events in adults but data on children are scarce. In the great majority of adult patients, thrombotic events are usually deep vein thrombosis and pulmonary embolism. Other sites such as jugular veins are extremely rare. We present a case of Lemierre syndrome in an adolescent girl with active ulcerative colitis and discuss possible risk factors. This is the first reported case of severe Lemierre syndrome with thrombus extension to cranial veins in a patient with ulcerative colitis. Early recognition of Lemierre syndrome in patients who present with rapidly worsening symptoms of neck pain, fever and signs of pharyngitis is imperative because it increases a chance of favorable prognosis. It is important for pediatricians treating IBD patients not to underestimate possible thrombotic events in children with IBD. Recognition of additional risk factors is crucial for prompt diagnosis and adequate treatment.
Inflammatory bowel disease (IBD) is a well-recognized risk factor for thrombotic events in the adult population [1]. Deep venous thrombosis and pulmonary embolism encounter for around 90% of all thromboembolic events in adults [1]. Other sites of thrombosis, like the jugular veins are extremely rare. Data on similar association in children is scarce [2].
Lemierre syndrome (LS) is a rare, potentially very serious condition characterized with thrombosis of the internal jugular vein (IJV) usually starting as oropharyngeal infection [3]. Septic emboli and thrombus can potentially extend to the central nervous system. The usual infectious pathogen is Fusobacterium necrophorum, and less frequently Fusobacterium nucleatum, anaerobic streptoccoci and other Gram-negative anaerobes [4].
We present a rare case of an adolescent girl with ulcerative colitis (UC) presenting with LS. To the best to our knowledge, this is the first such report in the literature.
A 17-year-old girl was admitted to a tertiary medical pediatric gastroenterology unit due to abdominal pain, fever, severe sideropenic anemia (hemoglobin level of 70 g/L and a ferritin value of 9 µg/L) and diarrhea. After extensive diagnostic work-up she was diagnosed with UC (pancolitis, E4 localization, Mayo score 2). She was treated with mesalamine and her symptoms and laboratory results improved. After 7 days of hospitalization she was discharged home. She was well for the first 9 days. However, she then developed fever (up to 38.7℃), sore throat, right neck swelling and tenderness. She was seen by her general practitioner who performed Group A streptococcal rapid antigen test which was negative. She was diagnosed with viral pharyngitis. In the next couple of days her symptoms worsened, the neck swelling enlarged and pain become severe (she could not move her head). On the 5th day of her symptoms she came to the emergency department of Children's Hospital Zagreb. Physical examination revealed right sided supraclavicular very painful edema, accompanied by reduced neck movement but without any skin changes. Her tonsils were red without exudate. Spleen was palpable 1 cm below the left costal margin, and the rest of the clinical examination was normal. Her family history was unremarkable for any chronic illness.
Laboratory results showed elevated leukocytes, elevated platelets count and inflammatory markers (Table 1). Coagulation test results were slightly out of the reference range (Table 1). Neck ultrasound revealed complete thrombosis of the right external and IJVs, 5 cm in length and a thrombus within right subclavian vein. Therefore, neck magnetic resonance imaginig (MRI) and MRI venography were performed and revealed thrombosis of the right subclavian vein, right jugular veins, truncus brachiocephalicus, partial thrombosis of the right sigmoid and transverse sinus (Fig. 1). Chest and heart ultrasound revealed bilateral pleural effusion and apical pericardial effusion with normal contractility of the heart.
Since the findings indicated the IJV thrombosis and right pleural effusion, and having taken into consideration the patient's medical history of pharyngitis, she was diagnosed with LS. The patient was immediately transported to the intensive care unit where she was treated with intravenous antibiotics (ceftriaxone and clindamycin for the first 5 days and then clindamycin for the following 9 days). She received subcutaneous low-molecular-weight heparin which was subsequently titrated depending on the anti-Xa activity.
Paired blood cultures and throat swabs had been taken before the antibiotic treatment was started but no pathogen was isolated.
She became afebrile after 72 hours of antibiotic treatment. She underwent comprehensive thrombophilia testing; factor V Leiden, protein C and S, factor VIII, and antithrombin III were found to be normal, antinuclear antibodies and anticardiolipin antibodies were negative and homocysteine levels were normal. Furthermore, F2 gene mutation G20210A c. *97G>A and FV Leiden c.1691G>A, p. Arg534GIn mutation were not found.
After complete stabilization, she was discharged from the hospital to continue anticoagulation therapy at home. Nine months after the hospitalization, low-molecular-weight heparin was replaced by the apixaban, peroral Xa inhibitor.
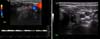
Subsequent follow-ups showed amelioration of neck edema. Neck ultrasound and color doppler performed on a monthly basis confirmed continuous regression of thrombosis and recanalization of the jugular vein (Fig. 2).
After discharge from the hospital, her UC was in clinical remission with (Paediatric Ulcerative Colitis Activity Index was 0) with normal calprotectin levels and no signs of anemia.
Even though it is well known that risk of thrombosis is increased in IBD, this is the first reported case of severe LS with thrombus extension to cranial veins in a patient with UC. Systematic review for thromboembolism in pediatric IBD showed that children with IBD have increased risk for thromboembolic events comparing to the healthy population and the risk is highest in children with active disease and with UC [5]. Some data show that the relative risk of thrombosis in IBD is in inverse proportion with age [6]. Risk factors in children are similar as in adults and include thrombocytosis, thrombocyte activation, hyperhomocysteinemia, increased fibrinogen and decreased fibrinolysis which are consequences of active inflammation [6]. Hereditary mutations of coagulation cascade and disease complications such as hospitalization, surgery, dehydration and vitamin deficiencies can further increase the risk [6]. Guidelines for the antithrombotic prophylaxis and prevention of thrombosis are extensively developed for adults with IBD, but there are no defined criteria for the juvenile population [7]. Our patient was comprehensively evaluated for congenital and acquired thrombophilia. She did not use any hormonal therapy (oral contraceptives) and her family history was unremarkable. Thrombophilia testing was found to be negative for all common established hereditary causes. Beside the still active UC at the time of thrombosis, we recognized other risk factors including recent hospitalization, pancolitis, anaemia, thrombocytosis and acute tonsillopharyngitis. These risk factors have been recognized as predisposing factors for overall thrombosis [8] but also risk factors for cerebral thrombosis [7]. It could be argued that association between LS and UC in our patient is only coincidence. However, LS is very rare disease in paediatric age and, despite rising incidence, UC is also infrequent, making this association as pure coincidence unlikely. Furthermore, as previously mentioned UC is a risk factor for thrombosis and our patient at the time of thrombotic event still had active disease which could attribute to the development of LS.
This illustrative case emphasizes the importance of early recognition of LS in patients who present with rapidly worsening symptoms of neck pain, fever and signs of pharyngitis. However, it has been shown in the literature that diagnosis is often delayed due to obscurity of signs and symptoms [39]. Previously microbiology was cornerstone for diagnosis; however, currently imaging techniques are far more accurate and provide faster results, enabling prompt treatment [310]. Treatment should include intravenous antibiotics and consideration of anticoagulation therapy [4]. Use of anticoagulation therapy is still controversial, with series of patients showing favorable prognosis only with antibiotic therapy [11]. However, most authors do agree that anticoagulation should be used in cases with thrombophilia, progression of the thrombosis or extension into the central nervous system [412].
In conclusion, it is important for pediatricians treating IBD patients not to underestimate possible thrombotic events in children with IBD. Recognition of additional risk factors is crucial for prompt diagnosis and adequate treatment.
Figures and Tables
References
1. Papay P, Miehsler W, Tilg H, Petritsch W, Reinisch W, Mayer A, et al. Clinical presentation of venous thromboembolism in inflammatory bowel disease. J Crohns Colitis. 2013; 7:723–729.

2. Nylund CM, Goudie A, Garza JM, Crouch G, Denson LA. Venous thrombotic events in hospitalized children and adolescents with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013; 56:485–491.

3. Alperstein A, Fertig RM, Feldman M, Watford D, Nystrom S, Delva G, et al. Septic thrombophlebitis of the internal jugular vein, a case of Lemierre's syndrome. Intractable Rare Dis Res. 2017; 6:137–140.

4. Cupit-Link MC, Nageswara Rao A, Warad DM, Rodriguez V. Lemierre syndrome: a retrospective study of the role of anticoagulation and thrombosis outcomes. Acta Haematol. 2017; 137:59–65.

5. Lazzerini M, Bramuzzo M, Maschio M, Martelossi S, Ventura A. Thromboembolism in pediatric inflammatory bowel disease: systematic review. Inflamm Bowel Dis. 2011; 17:2174–2183.

6. Alkim H, Koksal AR, Boga S, Sen I, Alkim C. Etiopathogenesis, prevention, and treatment of thromboembolism in inflammatory bowel disease. Clin Appl Thromb Hemost. 2017; 23:501–510.

7. Van Assche G, Dignass A, Bokemeyer B, Danese S, Gionchetti P, Moser G, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis. 2013; 7:1–33.

8. Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol. 2007; 102:174–186.

9. Habek M, Petravić D, Ozretić D, Brinar VV. Horner syndrome due to jugular vein thrombosis (Lemierre syndrome). J Neurol Neurosurg Psychiatry. 2008; 79:293.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download