Abstract
Most solitary gastrointestinal (GI) polyps in children are either inflammatory or hamartomatous. Solitary hyperplastic polyp, sentinel polyp and solitary adenomatous polyp have been occasionally diagnosed in adults, but very rarely reported in Korean children. We recently came across a case with adenomatous polyp in the colon, a case with hyperplastic polyp beneath the gastroesophageal junction, a case with hyperplastic polyp in the prepyloric area, and a case with sentinel polyp in the distal esophagus, which are unusual pathologic types in children. These mucosal lesions were diagnosed incidentally during elective endoscopic examinations for GI symptoms. Most polyps do not cause significant symptoms, so the diagnosis might be delayed, especially in children, in whom GI endoscopy is not commonly performed for screening purpose as in the adults.
A solitary polyp in the upper gastrointestinal (UGI) tract is rarely seen in children. We have occasionally come across glycogenic acanthosis, papilloma, and inflammatory nodules in the esophagus in children. These esophageal polypoid lesions have little correlation to the patient's chief complains of GI symptoms, and most cases are incidentally detected during endoscopy. Sentinel polyp, a kind of inflammatory polyp in the esophagus is not rare in adults, but very few cases have been reported in the pediatric population.
Juvenile polyp is the most common colon polyp in children, comprising more than 90% of all pediatric colonic polyps; it may be solitary or multiple [1]. This polyp is known to be benign and does not tend to be malignant. However, there are some case reports about juvenile polyps with adenomatous glands or hyperplastic glands, progressing to colon neoplasia [2]. Solitary adenomatous colon polyp has a high potential for malignancy, and is very rare in childhood. Hence, pathological investigation of a GI polyp is very important.
Authors recently came across several cases of colonic adenomatous polyp, gastric hyperplastic polyp (HPP), and esophageal sentinel polyp in children, discovered incidentally and diagnosed using pathological examination.
A 15-year-old girl presented with recurrent abdominal pain for 6 years. Initial laboratory workup revealed a white blood cell count (WBC) of 5,000/mm3 with 66% neutrophils, hemoglobin level of 12.7 g/dL, and platelet count of 202×103/mm3. Electrolytes levels showed sodium 143 mmol/L, potassium 4.6 mmol/L and chloride 108 mmol/L. The total protein level was 7.0 g/dL, albumin 4.1 g/dL, calcium 9.1 mg/dL, phosphorus 3.5 mg/dL, cholesterol 123.5 mg/dL, glucose 91 mg/dL, blood urea nitrogen (BUN) 7.7 mg/dL, creatinine 0.69 mg/dL, total bilirubin 0.50 mg/dL, aspartate aminotransferase (AST) 15 U/L, alanine aminotransferase (ALT) 7 U/L, amylase 52 U/L, lipase 52 U/L, erythrocyte sedimentation rate (ESR) 2 mm/hour, and C-reactive protein (CRP) <0.3 mg/dL. Trace elements panel was normal. UGI endoscopy showed gastric ulcer in the prepyloric area, verrucous gastritis in the body, and reflux esophagitis of grade A. Colonoscopy revealed no definite mucosal lesion, but a solitary polyp was noted in the rectum. Additionally, multiple cystic lesions were found on pelvic ultrasonography, but these were not considered the direct cause of her chronic abdominal pain. Medical treatment was administered for her UGI mucosal lesions. The rectal polyp was removed using cold snare. The size of polyp was 0.5×0.3×0.2 cm. Pathological examination of the excised polyp revealed that it was a tubular adenomatous type, which is known to be a precancerous lesion (Fig. 1A). Her abdominal symptoms improved after polypectomy, although her chronic GI symptoms did not seem to be related to the polyp. Following the diagnosis, tumor marker tests (CA-125, αFP, β-HCG) were performed, and they were normal. The follow-up colonoscopy was performed in one year showed normal mucosal finding in the area of the previous polypectomy.
A 13-year-old boy presented with abdominal pain and diarrhea. Laboratory examination revealed mild leukocytosis of 10,800/mm3 with polymorphonuclear cell 81%, hemoglobin level of 14.5 g/dL and platelet count of 174×103/mm3. Electrolyte levels were sodium 137 mmol/L, potassium 3.7 mmol/L and chloride 98 mmol/L. The total protein level was 7.5 g/dL, albumin 4.6 g/dL, calcium 9.3 mg/dL, phosphorus 4.5 mg/dL, cholesterol 154.1 mg/dL, glucose 105 mg/dL, BUN 11.9 mg/dL, creatinine 0.80 mg/dL, total bilirubin 1.38 mg/dL, AST 29 U/L, ALT 30 U/L, amylase 42 U/L, lipase 26 U/L, ESR 20 mm/hour, and CRP 19.19 mg/dL. The urinary ketone was 4+. On stool examination, occult blood and leukocyte were positive. Abdominal X-ray showed mild gaseous dilatation of the small bowel and abdominal ultrasonography showed reactive lymph nodes at ileocecal area, with mild splenomegaly. He was hospitalized and administered fluid therapy, but the GI symptoms did not subside. The stool was positive for Campylobacter coli, which was considered the cause of his diarrhea. It was resolved after therapy. UGI endoscopy was performed for his continuing upper abdominal pain. Acute gastritis and duodenitis were noted, and a solitary non-pedunculated polyp with hypervascularity was found beneath the gastroesophageal junction (GEJ) area. The biopsy sample of the polyp indicated that it was hyperplastic type. There was no difference in the size and external feature of the polyp on follow-up endoscopy 6 months later. Polypectomy was performed. The size of polyp was 1.0×0.9×0.8 cm. The post-polypectomy pathological finding was the same as that seen earlier (Fig. 1B). He was treated with medicines of his acute gastritis and duodenitis for about 2 months and his UGI symptoms did not recur. Follow-up UGI endoscopy was not performed yet.
An 18-year-old girl presented with fatigue, anorexia, indigestion, constipation, and postprandial abdominal pain. She was diagnosed with cecal diverticulosis 5 years earlier, and Helicobacter pylori (HP)-positive gastric ulcer 1 year earlier. A previous UGI endoscopic examination showed no polyp in her stomach. On laboratory examination, WBC was 8,200/mm3, with polymorphonuclear cell 65%, hemoglobin 13.6 g/dL, and platelet count 205×103/mm3. Electrolytes levels showed sodium 142 mmol/L, potassium 4.4 mmol/L and chloride 106 mmol/L. The protein level was 7.4 g/dL, albumin 4.7 g/dL, calcium 9.3 mg/dL, phosphorus 4.7 mg/dL, cholesterol 167.9 mg/dL, glucose 99 mg/dL, BUN 10.1 mg/dL, creatinine 0.77 mg/dL, total bilirubin 0.35 mg/dL, AST 14 U/L, ALT 5 U/L, ESR 10 mm/hour, and CRP <0.3 mg/dL. UGI endoscopy revealed nodular gastritis in the antrum and a polyp of sessile type in the prepyloric area. Polypectomy was performed. The size of the polyp was 0.8×0.6×0.5 cm. She was negative to HP at the time of polypectomy. The pathological finding was HPP (Fig. 1C). She was treated with medicines for her UGI mucosal lesions and the previous GI symptoms were resolved. Follow-up UGI endoscopy would be performed in one year.
A 14-year-old girl presented with intermittent abdominal pain for 3 to 4 years, which had recently aggravated. Her parents had a history of HP-induced gastric ulcer and had been treated a few years ago. However, medical evaluation of the child had not been performed. Routine laboratory examination revealed WBC 7,200/mm3, with polymorphonuclear cell 55.5%, hemoglobin level 14.0 g/dL, and platelet count 213×103/mm3. Electrolytes showed sodium levels 140 mmol/L, potassium 3.4 mmol/L and chloride 105 mmol/L. The total protein level was 8.0 g/dL, albumin 4.8 g/dL, calcium 9.8 mg/dL, phosphorus 4.0 mg/dL, cholesterol 163 mg/dL, glucose 83 mg/dL, BUN 6.4 mg/dL, creatinine 0.62 mg/dL, total bilirubin 0.97 mg/dL, AST 17 U/L, ALT 9 U/L, and CRP <0.8 mg/dL. UGI endoscopy showed chronic superficial gastritis of the stomach body, marked hyperemia and a short mucosal breakage in the distal esophagus. A longitudinal sessile polyp was noted in the GEJ area. The polyp was markedly congested and appeared like a hemangioma. She was negative for HP in the rapid urea test, stool HP antigen, and serum HP-specific immunoglobulin G tests. The pathological finding was sentinel polyp (Fig. 1D). Polypectomy has not been performed yet. He is taking anti-reflux medicine, and no specific GI symptom presents. Follow-up UGI endoscopy would be performed in one year.
GI polyps in children are usually juvenile polyps, familial adenomatous polyposis coli, Peutz-Jeghers syndrome (PJS), Cowden syndrome, and Bannayan-Riley-Ruvalcaba syndrome. Of these, juvenile polyps are most common.
The most important clinical issue of the GI polyps is whether the lesion has precancerous nature. In adults, fundic gland polyp, HPP, adenomatous polyp, and hamartomatous polyp as a manifestation of juvenile polyposis syndrome, PJS, Cowden's syndrome are solitary epithelial gastric polyps. These polyps are solitary or multiple. Among these, the potential for malignancy is highest in adenomatous polyp. Hyperplastic polyp has a low tendency for malignancy but evaluated to be significant [3]. Cancerous changes in hyperplastic gastric polyps have been suspected when the surrounding abnormal areas are related to the development of cancer elsewhere in the stomach [4]. The possibility of malignant change in the polyp is of clinical significance.
A previous case study by Lim et al. [5] reported 37 colonic polyps in Korean children. Of these, familial adenomatous polyposis was noted in 6, but no solitary adenomatous polyp was found. There are few case reports of solitary adenomatous polyp in Korean children. Kim et al. [6] reported a case of isolated adenomatous polyp in a 4-year-old toddler, who presented with occasional fresh rectal bleeding just after defecation for 4 months. This was quite different from our patient who had no rectal bleeding. A case of Peutz-Jegher syndrome with an intestinal adenomatous polyp was reported in a 15-month-old baby in Korea [7].
The major cause for gastric HPP is HP infection and long-term use of proton pump inhibitor (PPI) as documented in a prospective study by Hongo et al. [8]. They showed that HPP developed in HP-positive patients with higher serum gastrin level, who were on PPI for 104 weeks. Globally, reports about gastric HPP are very few. In 2002, a retrospective study revealed that gastric HPP developed in 4 out of 31 children who were on long-term omeprazole therapy for gastroesophageal (GE) reflux, and all polyps were found in the gastric body [9]. Gastric HPP is related to HP infection, and might resolve after HP treatment [10]. However, it is paradoxical that gastric HPP developed 1 year after HP treatment in our case 3. It is uncertain whether this polyp was due to HP infection or PPI. In our institution PPI is not prescribed for more than 4 weeks; hence PPI as the cause of HPP is less likely.
Gastric hyperplastic tumor is less likely to progress to malignancy than adenomatous polyp [11]. However, there have been several case reports about adenocarcinoma originating from gastric HPP [1213]. Additionally, some cases of signet ring cell carcinoma due to previous HPP infection were reported [14]. The Korean patient with signet ring cell carcinoma was diagnosed 17 months after the previous endoscopic diagnosis of HPP. The size of cancerous polyp increased two times, from 1 to 2 cm, and it was positive to pan-cytokeratin, CK-7, CEA, and MUC-1 on immunohistochemistry studies. Han et al. [15] investigated conditions in which cancerous pathologic changes were more frequent in gastric HPPs in 269 polypectomy samples from adult patients, and reported that neoplastic changes were more frequent if the polyp size was >1 cm [15].
Sentinel polyp is an inflammatory polyp-fold complex, originating from the cardiac portion of GEJ. This polyp is a kind of secondary inflammation accompanied with erosive esophagitis. The pathological finding is HPP consisting of inflammatory infiltrates and granulomatous tissue. This polyp is thought to be related to GE reflux; however, it does not resolve even after GE reflux is treated [16]. Our case did not show any endoscopic finding of reflux-associated esophageal mucosal lesion, such as breakage in the distal esophagus mucosa, GE reflux phenomenon on imaging, and the patient had no symptoms related to GE reflux. Thus, the reason for development of sentinel polyp in this case is unknown. Sentinel polyp is not rare in adults, but pediatric cases have been very rarely reported. Four cases were reported by Bishop et al. [17] in 2002, all of which were related to GE reflux. Two of these showed decrease in size and pathologic improvement after anti-reflux therapy [17]. This improvement in the size and the pathology is quite distinct.
The four cases of solitary polyps were small polyps and did not seem to be directly related to the GI symptoms, but they might be pathologically significant. Periodic, scheduled GI endoscopic examinations of screening purposes are popular among the adults in Korea. However, such scheduled examination is not possible in children. The above case with adenomatous polyp was incidentally diagnosed during investigations for the GI symptoms. General awareness about the need and importance of periodic GI endoscopic examination is necessary. Because all these cases are very recently diagnosed ones, the long term follow-up mucosal findings would be performed one or two years later.
Figures and Tables
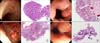 | Fig. 1(A) Left: a solitary, non-pedunculated polyp with smooth surface in the rectum. Right: the polyp shows proliferation of irregular dysplastic glands (H&E stain, ×100). (B) Left: there is a non-pedunculated polyp with rough and markedly congested surface beneath the gastroesophageal junction (GEJ) area. Right: the polyp shows hyperplastic foveolar glands, suggestive of hyperplastic polyp (H&E stain, ×100). (C) Left: there is a non-pedunculated polyp with moderate congestion and coarse surface in the prepyloric area. The surrounding antral mucosa is nodular and edematous. Right: the polyp shows hyperplastic foveolar glands, suggestive of hyperplastic polyp (H&E stain, ×100). (D) Left: there is a longitudinal, broad-based polyp with a smooth surface in the GEJ region. Right: the biopsy reveals hyperplastic foveolar glands, suggestive of hyperplastic polyp (H&E stain, ×100). |
References
1. Horrilleno EG, Eckert C, Ackerman LV. Polyps of the rectum and colon in children. Cancer. 1957; 10:1210–1220.

2. Friedman CJ, Fechner RE. A solitary juvenile polyp with hyperplastic and adenomatous glands. Dig Dis Sci. 1982; 27:946–948.

3. Goddard AF, Badreldin R, Pritchard DM, Walker MM, Warren B. on behalf of the British Society of Gastroenterology. The management of gastric polyp. Gut. 2010; 59:1270–1276.
4. Dirschmid K, Platz-Baudin C, Stolte M. Why is the hyperplastic polyp a marker for the precancerous condition of the gastric mucosa? Virchows Arch. 2006; 448:80–84.

5. Lim MS, Seo JK, Ko JS, Yang HR, Kang GH, Kim WS. Clinical, endoscopic and pathologic findings of colonic polyposis in Korean children. Korean J Pediatr Gastroenterol Nutr. 2010; 13:154–163.

6. Kim JW, Song JY, Lee HK, Yun HS, Yang I, Shim JW, et al. A case of isolated adenomatous polyp of rectum in a child: tubulovillous adenoma. Korean J Pediatr Gastroenterol Nutr. 1999; 2:250–255.

7. Lee KS, Lee SH, Myong NH. Peutz-Jeghers syndrome with adenomatous change in a fifteen-month-old boy. Korean J Gastroenterol. 2015; 66:106–110.

8. Hongo M, Fujimoto K. Gastric Polyps Study Group. Incidence and risk factor of fundic gland polyp and hyperplastic polyp in long-term proton pump inhibitor therapy: a prospective study in Japan. J Gastroenterol. 2010; 45:618–624.

9. Pashankar DS, Israel DM. Gastric polyps and nodules in children receiving long-term omeprazole therapy. J Pediatr Gastroenterol Nutr. 2002; 35:658–662.

10. Ohkusa T, Takashimizu I, Fujiki K, Suzuki S, Shimoi K, Horiuchi T, et al. Disappearance of hyperplastic polyps in the stomach after eradication of Helicobacter pylori. A randomized, clinical trial. Ann Intern Med. 1998; 129:712–715.

11. Nakamura T, Nakano G. Histopathological classification and malignant change in gastric polyps. J Clin Pathol. 1985; 38:754–764.

12. Park SJ, Kim HG, Yang JH, Song HJ, Jin SY, Ryu CB, et al. A case of adenocarcinoma arising in hyperplastic polyp in the stomach. Korean J Gastrointest Endosc. 2003; 27:153–157.
13. Fry LC, Lazenby AJ, Lee DH, Mönkemüller K. Signet-ring-cell adenocarcinoma arising from a hyperplastic polyp in the stomach. Gastrointest Endosc. 2005; 61:493–495.

14. Ryu HS, Shin SR, Kim KH, Seo GS, Choi SC. A case of a gastric hyperplastic polyp with a signet ring cell carcinoma. Korean J Gastrointest Endosc. 2008; 36:376–379.
15. Han AR, Sung CO, Kim KM, Park CK, Min BH, Lee JH, et al. The clinicopathological features of gastric hyperplastic polyps with neoplastic transformations: a suggestion of indication for endoscopic polypectomy. Gut Liver. 2009; 3:271–275.

16. Abraham SC, Singh VK, Yardley JH, Wu TT. Hyperplastic polyps of the esophagus and esophagogastric junction: histologic and clinicopathologic findings. Am J Surg Pathol. 2001; 25:1180–1187.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download