Abstract
Background and Purpose
Sudden neurological deterioration which cannot be explained by structural change, ischemia or seizure is often observed among neurosurgical patients. We aimed to provide new insight into the pathophysiology of postoperative transient neurologic dysfunction.
Methods
We describe prolonged but fully reversible focal neurologic dysfunction of unknown origin based on the initial evaluation in 8 patients who had received encephalo-duro-arterio-synangiosis for moyamoya disease. We performed brain imaging, including diffusion weighted imaging and perfusion magnetic resonance imaging or single photon emission computed tomography, and electroencephalography (EEG) during the episodes and after resolution of the symptoms.
Results
The symptoms consisted of dysarthria, hemiparesis, or hemiparesthesia of limbs contralateral to the operated side. These symptoms developed between 12 hours and 8 days after surgery and lasted between 12 hours and 17 days. Structural imaging did not show any significant interval change compared with the immediate postoperative images. Perfusion imaging showed increased cerebral blood flow in the symptomatic hemisphere. EEG revealed low amplitude arrhythmic slowing in the corresponding hemisphere. Follow-up imaging and EEG after recovery did not show any abnormalities.
Conclusions
Transient neurologic dysfunction can occur during the postoperative period of brain surgery. Although this may last more than usual transient ischemic attack or seizure, it eventually resolves regardless of treatment. Based on our observation, we propose that this is the manifestation of the transient cortical depression triggered by mechanical stimulation, analogous to migraine aura associated with cortical spreading depression.
Sudden neurological deterioration without any correlating structural cause is often observed in neurosurgical patients. However, this has been commonly overlooked or assumed to have been due to hemorrhage, ischemia, brain swelling or seizure, albeit lacking evidence. Typically, the patients eventually recover, which could be misinterpreted as the effect of a particular treatment for a presumed diagnosis. This is one of the reasons that the pathophysiology remains uncertain. Challenges in conducting sufficient diagnostic evaluation in such a critical condition would be another.
We present the cases with prolonged reversible neurological dysfunction which occurred during the postoperative period of indirect revascularization surgery for moyamoya disease as an initial study, because we could perform comprehensive workup to rule out other possible causes and elucidate the pathophysiology in these patients. We found that lateralized slowing of electroencephalography (EEG) and regional hyperperfusion in the absence of any structural lesions were the characteristics of this phenomenon.
We hypothesize that transient cortical depression occurs as a response to noxious stimuli to the cerebral cortex and is responsible for this transient neurologic dysfunction (TND). Leão1 demonstrated that focal stimulation of the rabbit cerebral cortex elicited cortical spreading depression (CSD), which has been suggested as a pathophysiology of migraine aura.2 Recently, there has been increasing evidence that electrocorticography (ECoG) changes resembling CSD occur in acutely injured human subjects,34567 whereas the clinical correlates of the electrophysiological phenomenon have yet to be elucidated.
In this study we report a novel category of transient focal neurologic dysfunction and elucidate the underlying mechanism.
We collected data from patients from two hospitals who had focal neurological dysfunction of unknown origin at the initial evaluation during the postoperative period of encephalo-duro-arterio-synangiosis (EDAS) for moyamoya disease. Detailed diagnostic and surgical procedures have been described in previous reports.8 Briefly, EDAS consists of splitting of a wide galeal flap and a superficial temporal artery from the scalp, performing craniotomy and creating a dural opening over the cerebral convexity, with partial opening of arachnoid membranes, and adjoining of the galeal flap onto the pial surface of the exposed cerebral convexity for angiogenesis.
Two children and six adults were included in the study. We performed diffusion weighted imaging (DWI) as the initial evaluation of the neurological dysfunction for all patients. Brain computed tomography (CT) was performed in six patients. Conventional magnetic resonance imaging (MRI) was performed in three patients. Brain perfusion single photon emission computed tomography (SPECT) with 99mTc-hexamethylpropyleneamine oxime (HMPAO) for the adult patients and perfusion MRI by dynamic susceptibility contrast method for the pediatric patients were performed to evaluate perfusion status. We checked scalp EEG in five patients during the episodes. EEGs were recorded over all brain areas using 19 electrodes for the children and 21 electrodes for the adults, according to conventional 10–20 system of electrode placement.
We repeated conventional MRI after the symptoms had resolved in all of the patients. Clinical characteristics, including symptoms and neurologic status during index events, symptoms associated with underlying moyamoya disease, and the type of surgery were also collected. This study was approved by Institutional Review Boards (IRBs) from both hospitals (IRB No. SNUH-1707-145-872, KNUH-2017-08-016-001, respectively). As a retrospective one, this study was exempted from obtaining individual patient's consent by IRBs. This manuscript follows the flow of CARE checklist (2013).
For better demonstration of the phenomenon, we shall first illustrate representative cases and then summarize the full clinical data.
This was an 11-year-old boy with moyamoya disease who had presented one month ago with sudden onset of headache and intraventricular hemorrhage. After stabilization and diagnostic work-up, he had received right hemispheric EDAS.
Twelve hours after surgery, he developed weakness of left arm and face. Neurologic examination demonstrated motor weakness of left hand (grade 1) and arm (grade 3), associated with headache. Rapid hydration was not effective and seven hours later he became drowsy. Brain CT and DWI showed mild brain swelling and a small amount of subdural hematoma (SDH) in right hemisphere (Supplementary Fig. 1 in the online-only Data Supplement). A second surgery was scheduled for decompression. When the bone flap and dura were opened, mild swelling of the brain and the overriding galeal layer was observed. However, the amount of SDH was negligible and thinning of bone flap to relieve pressure on the galeal flap and brain was performed. He became alert postoperatively and his motor power improved to grade 4.
Seventy-two hours after the initial surgery, he developed recurrent weakness of the left hand and arm (grade 1 and 3). Both Brain CT and DWI did not show any significant changes which could account for his hemiparesis (Supplementary Fig. 1 in the online-only Data Supplement). Perfusion MRI performed two days after the onset of the second symptom showed a subtle increase in cerebral blood volume (CBV) and cerebral blood flow (CBF) and shortening of time to peak (TTP) in the right frontal regions compared with the preoperative perfusion MRI (Fig. 1 and Supplementary Fig. 2 in the online-only Data Supplement). EEG performed on the same day showed continuous low amplitude arrhythmic mixed slowing in right hemisphere (Fig. 2). His motor function gradually improved from the fifth day onwards after the onset of his second attack. On the tenth day post-onset, his neurologic exam, brain MRI, and EEG were all normal. Perfusion MRI showed a decrease in CBV and CBF and lengthening of TTP in the right frontal regions compared with that performed during the second episode (Fig. 1 and Supplementary Fig. 2 in the online-only Data Supplement).
A 36-year-old female underwent right EDAS due to moyamoya disease. She initially presented with a transient ischemic attack (TIA) manifesting as left hemiparesis and hypesthesia. On the fourth day after surgery, she developed left hemiparesis and hypesthesia. There was only a small amount of SDH in right hemisphere on DWI (Supplementary Fig. 3 in the online-only Data Supplement). On brain SPECT performed seven days after the onset, an increased uptake of HMPAO was identified in the right hemisphere (Fig. 3). EEG conducted on the same day showed continuous low amplitude arrhythmic theta slowing in the right hemisphere (Fig. 3). Initially, hydration and inotropics were administered under the suspicion of TIA. After identifying the hyperperfusion on SPECT, normohydration and normotension were maintained. She gradually improved since the fifth day from the onset and the patient completely recovered on the tenth day without any sequelae.
Data from all eight patients are summarized in Table 1. Their preoperative symptoms consisted of transient hemiparesis, hemiparesthesia, or hemianopia except for patient 1, who had sudden onset of headache due to intraventricular hemorrhage. Three of them had cerebral infarction. All were diagnosed with moyamoya disease based on the typical symptoms and angiographic findings.
The postoperative symptoms were dysarthria, hemiparesis, or hemiparesthesia of the limbs contralateral to the operation site. The symptoms were quite different from the preoperative symptoms in all patients except patient 4. A brief transient symptom preceded the prolonged episode in two patients (patient 1 and 2) and the symptom wax and waned repeatedly in another patient (patient 6). One had a focal motor seizure in the paretic arm for 3 minutes during the episode (Case 2). All of the symptoms occurred between 12 hours and 8 days after surgery and lasted from 12 hours to 17 days. The onset was sudden and the improvement was mostly gradual. Otherwise, all patients were in good postoperative condition and had no postoperative complications.
Brain structural imaging showed only minimal subdural hemorrhage associated with surgery without any interval changes. Perfusion imaging showed a modest increase in CBF in the symptomatic hemisphere, compared with the preoperative study. EEG did not show any epileptiform discharges but revealed persistent arrhythmic mixed slowing in symptomatic hemisphere. The amplitudes on the EEG were comparable to or lower on the symptomatic side.
No improvement was achieved by saline loading, which was attempted based on the speculation that the ischemia might have been associated with dehydration. In some of the adult patients, inotropics were also initiated to increase perfusion but discontinued after hyperperfusion was suspected on SPECT. All patients were started on prophylactic levetiracetam postoperatively. Oxcarbazepine was started in a patient who had a seizure, which did not improve the hemiparesis (Case 2). All patients fully recovered eventually. Brain MRIs performed after recovery also did not show any abnormalities other than preexisting lesions. EEGs became normal and perfusion MRIs returned to their preoperative state.
We presented eight cases which shared common clinical features in the postoperative setting of EDAS. This was characterized by transient focal neurological hypoactivity, electrical depression, and hyperperfusion. It is contradistinctive of TIA and seizures: TIAs are characterized by hypoactivity, electrical depression, and hypoperfusion, whereas seizures are accompanied by hyperactivity, electrical hypersynchrony, and hyperperfusion.
Considering the nature of moyamoya disease and its common postoperative complications, intracranial bleeding such as SDH or ischemia would have been the most plausible cause for the observed neurological deterioration. However, we were unable to find any degree of significant hematoma to explain the deficits, nor were there any definite diffusion-restricted lesions. The semiology was different from the patients' preoperative TIAs, and it persisted longer than the typical duration of a TIA. Moreover, we ruled out the possibility of a prolonged TIA by showing no decrease in CBF involving the corresponding hemisphere. Non-convulsive seizures were also less likely as there were no evident epileptiform discharges on EEG performed during the episode. The surgical region was away from the site where a negative motor seizure could arise. One child had an overt motor seizure, but the entire episode could not be explained on the basis of a post-ictal Todd's paralysis as the weakness preceded the seizure attack and lasted for 16 days after a seizure which only lasted 3 minutes.
A close similarity exists between this phenomenon and hemiplegic migraine with regard to semiology, EEG depression, and hyperperfusion. Migraine is a prototypical paroxysmal disorder. CSD was suggested as a pathophysiology of migraine, based on the similarity between the typical pattern of symptom evolution of a migraine aura and the propagation of CSD triggered in animal brains, in spite of the absence of direct evidence for humans.19 Later, genetic mutations in ion channels in familial hemiplegic migraine supported the link between CSD and migraine.10 EEGs during migraine attacks usually show attenuation or slow activity in the symptomatic hemisphere, although a ‘spreading’ pattern of depression has not been documented. Most cases showed vasodilation or hyperperfusion during a migraine aura on vascular or perfusion imaging.11
In our patients, EEG showed slowing or attenuation during the TND episodes, despite the fact that EEG amplitudes are usually higher post-craniotomy. As for the observed hyperperfusion in symptomatic hemisphere, there could be several explanations besides hyperemia associated with CSD. Nonspecific hyperemia induced by surgical manipulation itself, regardless of the symptomatology, is a possibility. Another possible explanation is a reperfusion response. There have been reports of TND after superficial temporal artery to middle cerebral artery (STA-MCA) bypass (frequently combined with EDAS) in moyamoya patients,1213 which have shown increased perfusion on the symptomatic side and hyperperfusion syndrome was assumed to have been the cause. The TND after STA-MCA bypass in general did not accompany significant edema or hemorrhage, which is typical in hyperperfusion syndrome occurring after carotid endarterectomy/stent. This was understood as STA-MCA bypass restores low flow whereas carotid endarterectomy/stent restores high flow perfusion.13 Previously, TND among several patients in this study had been reported under the assumption of symptomatic hyperperfusion.14 However, as angiogenesis requires time and it is unlikely that rapid reperfusion occurs after indirect revascularization such as EDAS, the pathophysiology of TND remained unanswered. Furthermore, we observed additional cases with recurrent TND during the postoperative period and seizures were ruled out by performing EEG during the episodes. Spontaneous resolution and recurrence do not support hyperperfusion as a cause of TND.
We propose that the transient cortical depression, namely, CSD triggered by mechanical stimuli, is the underlying mechanism of postoperative TND. CSD may spontaneously occur in those genetically predisposed to migraines but could also be provoked by acute brain injury in patients without having a history of migraines, just as seizures may arise in patients with epilepsy whereas an acute symptomatic seizure can be provoked by an acute brain insult. Migraines and seizures may coexist in a patient, such as seen in Case 2.15
However, it is unclear whether TND with electrophysiological dysfunction resembling CSD can develop in all neurosurgical patients, regardless of the underlying diseases and the types of operation. TND without any underlying structural lesions has been reported to occur after mild traumatic brain injury (TBI) as well. In some patients, the symptoms resembled an attack of hemiplegic migraine experienced by the patients or their relatives. Classic migraine with visual aura precipitated by head-on collision while playing football has also been reported. Therefore, this was referred to as traumatriggered migraine, post-traumatic migraine, or footballer's migraine.161718 We often observed prolonged but spontaneously recovering hemiparesis and/or dysphasia without any evidence of structural changes, ischemia/vasospasm, or seizures in the patients with acute subdural hemorrhage and aneurysmal subarachnoid hemorrhage regardless of whether surgery had been performed or not (unpublished). The common features of TBI, subarachnoid hemorrhage (SAH), and EDAS are that they all involve large areas of the cerebral cortex, including the sensorimotor areas, and may be associated with SAH, which could be the reason why this occurs rather frequently. Whereas CSD is more difficult to elicit in human than in rodent cortex,9 ECoG finding resembling CSD was reported not only in TBI but also in spontaneous intracerebral hemorrhage, SAH, and ischemic stroke.34567 Further studies correlating clinical symptoms and electrophysiologic findings in a broader clinical context are required.
Despite the symptoms being prolonged, all of our patients fully recovered without any sequalae as seen in TND after mild TBI, whereas previous reports of severe TBI and stroke suggested that the presence of prolonged CSD was associated with unfavorable clinical outcome.35 We speculate that CSD may not be due to severe pathology, nor mean necessarily that the prognosis is poor, but is rather an epiphenomenon associated with cortical stimulation.
Our results have important clinical implications. Sudden neurologic deterioration after surgery is a concern for clinicians. In patient 1, it was unclear whether the second surgery for decompression was truly beneficial. The brain swelling in CT was equivocal, although moderate brain swelling was observed in the surgical field. By understanding the nature of this phenomenon and its self-limiting course, clinicians can avoid unnecessary surgery or intervention if other causes necessitating urgent management have been ruled out. EEG can be quite useful in detecting seizures and monitor neurological status in cases where there is no evident hematoma or infarction. Treatment methods which can resolve or shorten the duration of this phenomenon are lacking. To date, there have been no treatment methods proven to be effective for abolishing migraine aura. However, certain medications such as calcium channel blockers, tricyclic antidepressants, and antiepileptic drugs have been reported to decrease the recurrence of migraine attacks. Studies on the benefits of prophylactic medications in neurosurgical patients would be of interest.
In conclusion, we report an unusual postoperative phenomenon with prolonged reversible neurological dysfunction after EDAS. The main features include hypofunction, electrical depression, and hyperperfusion which differentiate this from ischemia or seizure, and suggest that there might be shared common pathophysiology with migraines. We propose ‘postoperative migraine’ evoked by mechanical stimulation on the cerebral cortex as the underlying mechanism of TND after EDAS.
References
1. Leão AA. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944; 7:359–390.
2. Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001; 81:1065–1096. PMID: 11427692.
3. Strong AJ, Fabricius M, Boutelle MG, Hibbins SJ, Hopwood SE, Jones R, et al. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke. 2002; 33:2738–2743. PMID: 12468763.

4. Mayevsky A, Doron A, Manor T, Meilin S, Zarchin N, Ouaknine GE. Cortical spreading depression recorded from the human brain using a multiparametric monitoring system. Brain Res. 1996; 740:268–274. PMID: 8973824.

5. Hartings JA, Bullock MR, Okonkwo DO, Murray LS, Murray GD, Fabricius M, et al. Spreading depolarisations and outcome after traumatic brain injury: a prospective observational study. Lancet Neurol. 2011; 10:1058–1064. PMID: 22056157.

6. Dohmen C, Sakowitz OW, Fabricius M, Bosche B, Reithmeier T, Ernestus RI, et al. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 2008; 63:720–728. PMID: 18496842.

7. Fabricius M, Fuhr S, Bhatia R, Boutelle M, Hashemi P, Strong AJ, et al. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006; 129:778–790. PMID: 16364954.

8. Cho WS, Kim JE, Kim CH, Ban SP, Kang HS, Son YJ, et al. Long-term outcomes after combined revascularization surgery in adult moyamoya disease. Stroke. 2014; 45:3025–3031. PMID: 25184359.

9. Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003; 4:386–398. PMID: 12728266.

10. Russell MB, Ducros A. Sporadic and familial hemiplegic migraine: pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol. 2011; 10:457–470. PMID: 21458376.

11. Choi KH, Kim JS, Lee SY, Ryu SW, Kim SS, Lee SH, et al. Familial hemiplegic migraine with prolonged coma and cerebellar atrophy: CACNA1A T666M mutation in a Korean family. J Korean Med Sci. 2012; 27:1124–1127. PMID: 22969264.

12. Fujimura M, Kaneta T, Shimizu H, Tominaga T. Symptomatic hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in a child with moyamoya disease. Childs Nerv Syst. 2007; 23:1195–1198. PMID: 17486352.

13. Kim JE, Oh CW, Kwon OK, Park SQ, Kim SE, Kim YK. Transient hyperperfusion after superficial temporal artery/middle cerebral artery bypass surgery as a possible cause of postoperative transient neurological deterioration. Cerebrovasc Dis. 2008; 25:580–586. PMID: 18483458.

14. Cho WS, Lee HY, Kang HS, Kim JE, Bang JS, Oh CW. Symptomatic cerebral hyperperfusion on SPECT after indirect revascularization surgery for moyamoya disease. Clin Nucl Med. 2013; 38:44–46. PMID: 23242046.

15. Rajapakse T, Buchhalter J. The borderland of migraine and epilepsy in children. Headache. 2016; 56:1071–1080. PMID: 27103497.

16. Haas DC, Lourie H. Trauma-triggered migraine: an explanation for common neurological attacks after mild head injury. review of the literature. J Neurosurg. 1988; 68:181–188. PMID: 3276835.
17. Sakas DE, Whittaker KW, Whitwell HL, Singounas EG. Syndromes of posttraumatic neurological deterioration in children with no focal lesions revealed by cerebral imaging: evidence for a trigeminovascular pathophysiology. Neurosurgery. 1997; 41:661–667. PMID: 9310985.

18. John Graham senior clinicians award lecture. posttraumatic migraine. Headache. 1998; 38:772–778. PMID: 11279902.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2018.14.3.393.
Supplementary Fig. 1
CT and DWI of patient 1 (Case 1) during the first and second episodes. At initial episode, CT showed a small amount of epidural and subdural hemorrhage at the operative field, but no diffusion restriction was noted on DWI. During the second episode, no structural abnormality explaining the neurological deficits was found. DWI: diffusion weighted imaging.
Supplementary Fig. 2
Perfusion MRI of patient 1 (Case 1). Mild increase of CBV was observed in the right frontoparietal area during the second episode. CBV was normalized after full neurological recovery. CBV: cerebral blood volume.
Supplementary Fig. 3
DWI with ADC map in patient 4 (Case 2). There was no diffusion restriction lesion in the operated brain area during the episode. ADC: apparent diffusion coefficient, DWI: diffusion weighted imaging.
Fig. 1
Perfusion MRI of patient 1 (Case 1) taken one week after the onset of intraventricular hemorrhage prior to surgery, on postoperative day 5 during the second episode of left arm weakness, and after recovery. A subtle increase in cerebral blood volume and CBF with shortening of TTP in the right frontal area was noticed during the second episode, compared with the images obtained at the preoperative period and after recovery from the episodes. CBF: cerebral blood flow, TTP: time to peak.
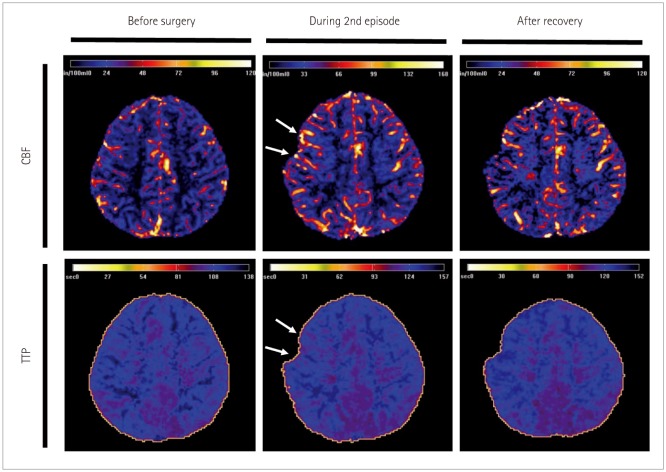
Fig. 2
Electroencephalography performed during the second episode of left arm weakness in patient 1 (Case 1). Continuous low amplitude arrhythmic mixed slowing was observed in the right hemisphere.
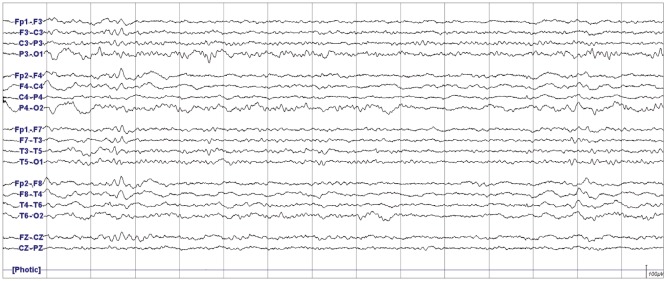
Fig. 3
Brain imaging and EEG tracings of patient 4 (Case 2). Left: single photon emission computed tomography performed during the preoperative and the postoperative period. There was an increase in perfusion in the right frontoparietal area during the episode compared with the preoperative period. Right: EEG performed during the episode shows continuous low amplitude arrhythmic mixed slowing was seen in the right hemisphere. EEG: electroencephalography.
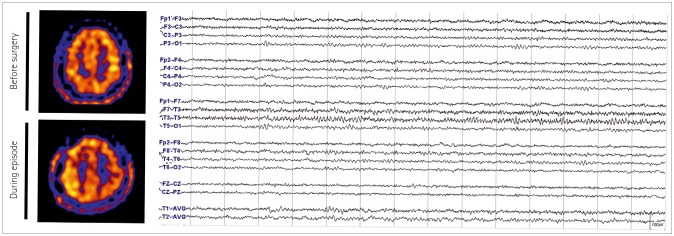
Table 1
Clinical characteristics of the subjects who developed transient neurological dysfunction of unknown origin at the initial evaluation during the postoperative period of indirect revascularization for moyamoya disease
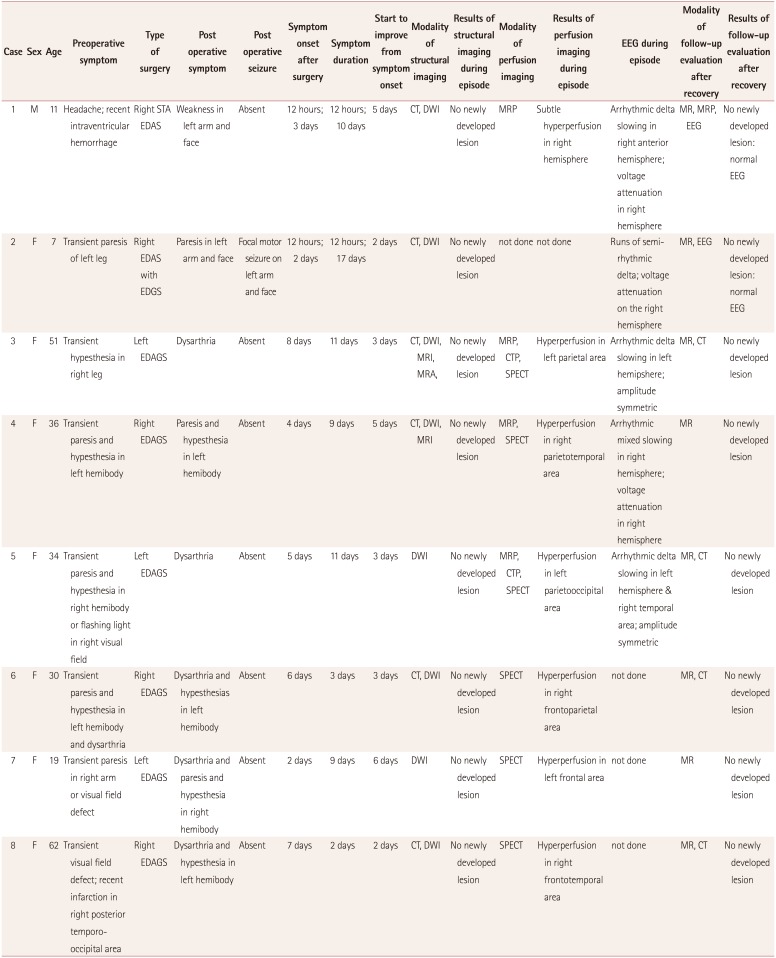
CT: computed tomography, CTP: computed tomography perfusion, DWI: diffusion weighted, EDAS: encephalo-duro-galeo-synangiosis, EDAGS: encephalo-duro-arterio-galeo-synangiosis, EDGS: encephalo-duro-galeo-synangiosis, EEG: electroencephalography, MR: magnetic resonance, MRA: magnetic resonance angiography, MRI: magnetic resonance imaging, MRP: magnetic resonance perfusion, SPECT: single photon emission computed tomography, STA EDAS: superficial temporal artery encephaloduro-arterio-synangiosis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download