This article has been
cited by other articles in ScienceCentral.
Abstract
Background and Purpose
Optic neuritis (ON) is an inflammation of the optic nerve that can be recurrent, with unilateral or bilateral presentation. Diagnosing recurrent cases may be challenging. We aimed to compare patients with recurrent ON as their initial symptom according to their following final diagnoses: multiple sclerosis (MS), neuromyelitis optica spectrum disorders (NMOSD), or chronic relapsing inflammatory optic neuropathy (CRION).
Methods
The medical records of patients with initial recurrent ON who were followed at the Neuroimmunology Clinic of the Federal University of São Paulo between 2004 and 2016 were analyzed retrospectively. Patients were classified according to their final diagnosis into MS, NMOSD, or CRION, and the characteristics of these groups were compared to identify predictive factors.
Results
Thirty-three patients with recurrent ON were included, and 6, 14, and 13 had final diagnoses of MS, NMOSD, and CRION, respectively. Most of the patients were female with unilateral and severe ON in their first episode, and the initial Visual Functional System Score (VFSS) was ≥5 in 63.6%, 85.7%, and 16.7% of the patients with CRION, NMOSD, and MS, respectively. Anti-aquaporin-4 antibodies were detected in 9 of 21 (42.8%) tested patients. Seven of nine (77.8%) seropositive NMOSD patients experienced transverse myelitis episodes during the follow-up period. A multivariate regression analysis showed that the VFSS at the last medical appointment predicted the final diagnosis.
Conclusions
A lower VFSS at the last medical appointment was predictive of MS. Patients with NMOSD and CRION have similar clinical characteristics, whereas NMOSD patients tend to have worse visual acuity.
Keywords: optic neuritis, multiple sclerosis, neuromyelitis optica, recurrence, aquaporin 4
INTRODUCTION
Optic neuritis (ON) is defined as demyelinating inflammation of the optic nerve and typically presents in association with multiple sclerosis (MS).
12 ON usually presents as a subacute unilateral and painful loss of vision that progresses over a few days to 2 weeks, and occasionally both eyes can be affected, either simultaneously or sequentially.
34 Symptoms include blurring of vision, reduced contrast sensitivity, dyschromatopsia, visual field defect and a relative afferent pupillary defect, and Uhthoff's phenomenon. Visible opticnerve inflammation on a funduscopic examination is present in about one-third of patients.
5 Despite careful diagnostic workup, in many cases the etiology of ON remains elusive at the first presentation, and 3–5% of patients experience recurrent episodes.
6
There is controversy about relapsing ON among different experts. This was initially considered a recurrent steroid-responsive optic neuropathy that differed from ON associated with demyelinating diseases, such as MS, in terms of the severity of visual loss, persistence of pain after the onset of visual loss, and recurrent episodes.
7 However, several recent studies found that 5–20% of patients with exclusive ON relapses tested positive for antiaquaporin-4 (anti-AQP-4) antibody, and roughly 50% of the seropositive patients develop longitudinally extensive transverse myelitis within 2 years.
28910111213
Relapsing ON that responds to corticosteroids or immunosuppressant treatment in patients who either do not fulfill MS diagnostic criteria or are negative for anti-AQP-4 antibody has recently been considered a disease in itself, called chronic relapsing inflammatory optic neuropathy (CRION).
12 However, there is still some controversy surrounding relapsing ON, especially its relationship to MS and neuromyelitis optica spectrum disorders (NMOSD).
To better understand the clinical characteristics of patients who present initially with exclusive demyelinating immune-mediated ON relapses, we reviewed our database with the aim of identifying the clinical, radiological, and serological features that would help distinguish between CRION, NMOSD, and MS.
METHODS
The Neuroimmunology Clinic of the Federal University of São Paulo is a specialized center for treating patients with MS and other demyelinating diseases. It maintains a database with information on the clinical status, results of laboratory tests and radiological evaluations, and treatment of all patients under follow-up since 1994. In December 2016 this database contained information on 1,823 registered patients, including 916 with a diagnosis of MS, 204 with isolated demyelinating syndrome, 185 with NMOSD, and 24 with relapsing isolated optic neuritis (RION).
This retrospective cohort study reviewed all medical records between February 1994 and December 2016, and patients were selected if they had experienced at least two clinical episodes of ON separated by at least 4 weeks. Patients with less than 6 months of follow-up, any evidence of systemic autoimmune, vascular, or infectious diseases, or evidence of disease activity in other areas of the central nervous system (CNS) prior to the ON were excluded (
Fig. 1). The study was approved by the Institutional Review Board of the Federal University of São Paulo as part of a long-term observational study on demyelinating diseases (IRB No. 933050).
Fig. 1
Diagram of the selection and inclusion criteria for the study. AION: anterior ischemic optic neuropathy, CRION: chronic relapsing inflammatory optic neuropathy, IDS: isolated demyelinating syndrome, MS: multiple sclerosis, NMOSD: neuromyelitis optica spectrum disorders, ON: optic neuritis, Others: other diseases, RION: relapsing isolated optic neuritis.
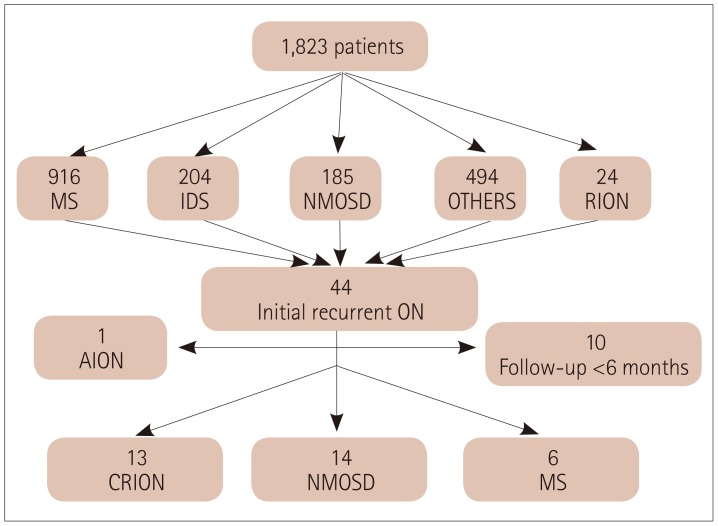

Relapse was defined as two clinical episodes of neurological symptoms separated by at least 4 weeks. ON was classified as bilateral if both eyes were involved simultaneously or sequentially within 4 weeks. RION was defined as a new unilateral or bilateral attack occurring after an interval of at least 4 weeks. The index event was defined as the first non-ON relapse after the first episode of ON. The patients were classified according to their final diagnosis in December 2016 as follows:
MS, if they fulfilled the clinical and imaging criteria for MS during follow-up.
14
NMOSD, if they fulfilled the revised clinical and imaging criteria for NMOSD during follow-up.
15
CRION, if they had RION but no imaging or clinical evidence of disease activity in other areas of the CNS and were receiving immunosuppressant or corticosteroid treatment to avoid further relapses or worsening of visual acuity (VA).
12
For each patient we recorded demographic data (sex, age at onset, and follow-up period), the medical history of each ON episode and visual disability, brain and spinal-cord magnetic resonance imaging (MRI) data, and laboratory data (e.g., cerebrospinal fluid and anti-AQP-4 antibody). Anti-AQP-4 antibody was evaluated using a commercially available indirect immunofluorescence method in all patients with RION who did not meet MS criteria since 2007, due to anti-AQP-4 antibody not being available in Brazil before this date. Patients with MS received the standard treatment with immunomodulatory drugs, while patients with NMOSD and CRION were treated with oral steroids and/or azathioprine as required.
A severe visual outcome was defined as VA ≤20/200. Clinical severity was evaluated by the Visual Functional System Score (VFSS) in the Expanded Disability Status Scale of Kurtzke. The VFSS ranges from 0 to 6, where 0 refers to normal VA and 6 to VA <20/200 in the worse eye and ≤20/60 in the best eye.
16 The VFSS was recorded at each visit, but only the scores at the first and last medical appointments were used in this analysis.
We considered that a VFSS of ≥5 indicated significant clinical disability due to its negative impact on the social life. To avoid bias due to the disease duration, we normalized the total number of ON relapses by the total disease duration (in years), thereby using the annualized relapse rate (aRR) to evaluate disease severity.
We performed a multinomial regression analysis to determine whether age, sex, type of ON (unilateral or bilateral), initial VA, initial VFSS, final VA, or final VFSS predicted the final diagnosis of MS, NMOSD, or CRION.
Statistical analysis was performed using the programs SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and STATA 14 (StataCorp LP, College Station, College Station, TX, USA). Categorical data were described as absolute values (n) and relative frequencies (%), while continuous and semicontinuous data were described as medians and interquartile ranges. The Mann-Whitney test was used to compare independent samples, while Fisher's exact test was used to compare categorical data. The Kaplan-Meier survival method and the log-rank test were used to compare times to VA ≤20/200 (monocular and binocular) between the NMOSD and CRION groups, and among the patients who were positive and negative for anti-AQP-4 antibody. A probability value of <0.05 was considered statistically significant.
RESULTS
Thirty-three patients with bilateral or unilateral relapsing ON were included, comprising 13 with a final diagnosis of CRION, 14 with NMOSD, and 6 with MS. The main clinical and demographic results are summarized in
Table 1.
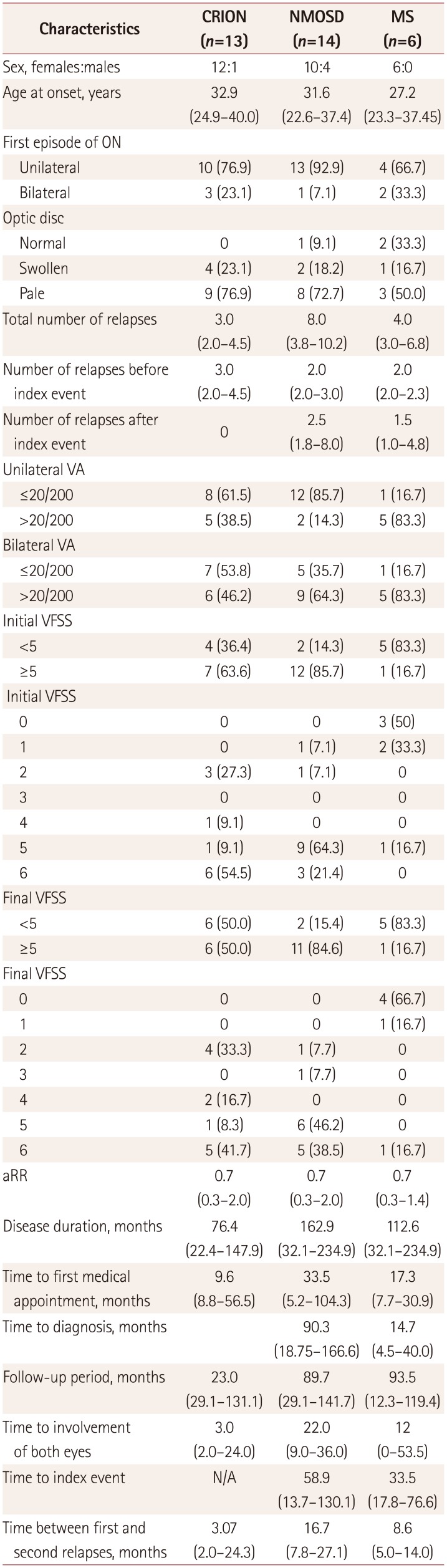
Table 1
Demographic and clinical characteristics of patients with CRION, NMOSD, and MS
|
Characteristics |
CRION (n=13) |
NMOSD (n=14) |
MS (n=6) |
|
Sex, females:males |
12:1 |
10:4 |
6:0 |
|
Age at onset, years |
32.9 (24.9–40.0) |
31.6 (22.6–37.4) |
27.2 (23.3–37.45) |
|
First episode of ON |
|
|
|
|
Unilateral |
10 (76.9) |
13 (92.9) |
4 (66.7) |
|
Bilateral |
3 (23.1) |
1 (7.1) |
2 (33.3) |
|
Optic disc |
|
|
|
|
Normal |
0 |
1 (9.1) |
2 (33.3) |
|
Swollen |
4 (23.1) |
2 (18.2) |
1 (16.7) |
|
Pale |
9 (76.9) |
8 (72.7) |
3 (50.0) |
|
Total number of relapses |
3.0 (2.0–4.5) |
8.0 (3.8–10.2) |
4.0 (3.0–6.8) |
|
Number of relapses before index event |
3.0 (2.0–4.5) |
2.0 (2.0–3.0) |
2.0 (2.0–2.3) |
|
Number of relapses after index event 0 |
2.5 (1.8–8.0) |
1.5 (1.0–4.8) |
|
|
Unilateral VA |
|
|
|
|
≤20/200 |
8 (61.5) |
12 (85.7) |
1 (16.7) |
|
>20/200 |
5 (38.5) |
2 (14.3) |
5 (83.3) |
|
Bilateral VA |
|
|
|
|
≤20/200 |
7 (53.8) |
5 (35.7) |
1 (16.7) |
|
>20/200 |
6 (46.2) |
9 (64.3) |
5 (83.3) |
|
Initial VFSS |
|
|
|
|
<5 |
4 (36.4) |
2 (14.3) |
5 (83.3) |
|
≥5 |
7 (63.6) |
12 (85.7) |
1 (16.7) |
|
Initial VFSS |
|
|
|
|
0 |
0 |
0 |
3 (50) |
|
1 |
0 |
1 (7.1) |
2 (33.3) |
|
2 |
3 (27.3) |
1 (7.1) |
0 |
|
3 |
0 |
0 |
0 |
|
4 |
1 (9.1) |
0 |
0 |
|
5 |
1 (9.1) |
9 (64.3) |
1 (16.7) |
|
6 |
6 (54.5) |
3 (21.4) |
0 |
|
Final VFSS |
|
|
|
|
<5 |
6 (50.0) |
2 (15.4) |
5 (83.3) |
|
≥5 |
6 (50.0) |
11 (84.6) |
1 (16.7) |
|
Final VFSS |
|
|
|
|
0 |
0 |
0 |
4 (66.7) |
|
1 |
0 |
0 |
1 (16.7) |
|
2 |
4 (33.3) |
1 (7.7) |
0 |
|
3 |
0 |
1 (7.7) |
0 |
|
4 |
2 (16.7) |
0 |
0 |
|
5 |
1 (8.3) |
6 (46.2) |
0 |
|
6 |
5 (41.7) |
5 (38.5) |
1 (16.7) |
|
aRR |
0.7 (0.3–2.0) |
0.7 (0.3–2.0) |
0.7 (0.3–1.4) |
|
Disease duration, months |
76.4 (22.4–147.9) |
162.9 (32.1–234.9) |
112.6 (32.1–234.9) |
|
Time to first medical appointment, months |
9.6 (8.8–56.5) |
33.5 (5.2–104.3) |
17.3 (7.7–30.9) |
|
Time to diagnosis, months |
|
90.3 (18.75–166.6) |
14.7 (4.5–40.0) |
|
Follow-up period, months |
23.0 (29.1–131.1) |
89.7 (29.1–141.7) |
93.5 (12.3–119.4) |
|
Time to involvement of both eyes |
3.0 (2.0–24.0) |
22.0 (9.0–36.0) |
12 (0–53.5) |
|
Time to index event |
N/A |
58.9 (13.7–130.1) |
33.5 (17.8–76.6) |
|
Time between first and second relapses, months |
3.07 (2.0–24.3) |
16.7 (7.8–27.1) |
8.6 (5.0–14.0) |

The female:male ratios were 12:1, 10:4, and 6:0 for CRION, NMOSD, and MS, respectively, and the corresponding median ages at onset were 32.9, 31.6, and 27.2 years. The first ON episode was unilateral in most patients regardless of the final diagnosis, with rates of 76.9%, 92.9%, and 66.7% for CRION, NMOSD, and MS, respectively. The disease duration, follow-up period, time to the index event, time between first and second relapses, and time to the involvement of both eyes did not differ between CRION, NMOSD, and MS. However, the interval between the first ON episode and the final diagnosis was shorter in MS than in NMOSD (14.7 months vs. 90.3 months, p=0.039).
The median number of relapses (including ON and other neurological topographies such as myelitis) was higher in patients with NMOSD, but the aRR was the same in the three groups (
Table 1). The initial VFSS was worse in CRION and NMOSD than in MS (
p=0.05 for CRION vs. MS, and
p=0.002 for NMOSD vs. MS), but did not differ between CRION and NMOSD (
p=0.52) (
Fig. 2). The final VFSS was also worse in CRION and NMOSD than in MS (
p=0.035 for CRION vs. MS, and
p=0.011 for NMOSD vs. MS) (
Fig. 2,
Table 2).
Fig. 2
A: Initial VFSS according to the diagnosis: p=0.05 for CRION vs. MS, p=0.002 for NMOSD vs. MS, and p=0.52 for CRION vs. NMOSD. B: Final VFSS according to the diagnosis. p=0.035 for CRION vs. MS and p=0.011 for NMOSD vs. MS. p<0.05, statistically significant difference between the groups. CRION: chronic relapsing inflammatory optic neuropathy, MS: multiple sclerosis, NMOSD: neuromyelitis optica spectrum disorders, VFSS: Visual Functional System Score.
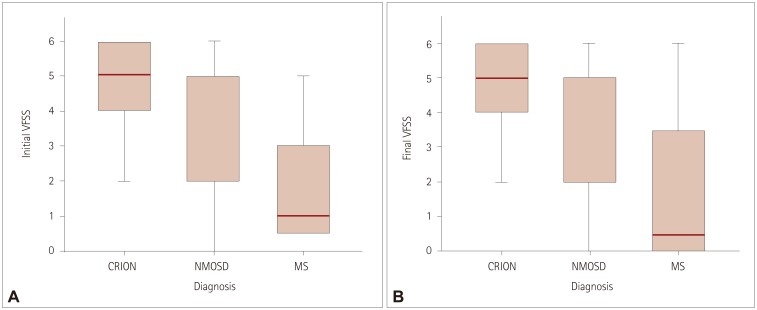

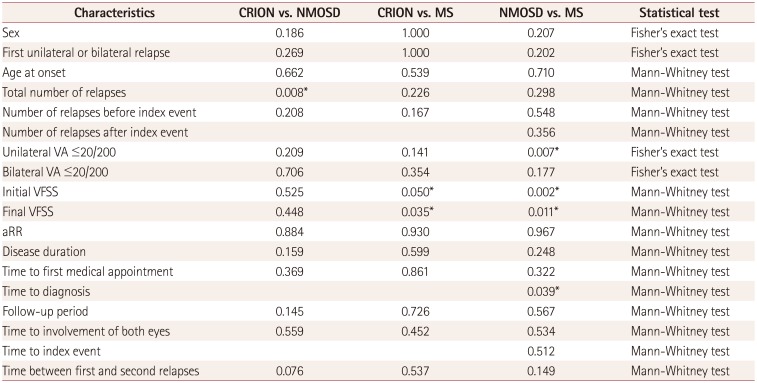
Table 2
Comparison of clinical characteristics between MS, NMOSD, and CRION
|
Characteristics |
CRION vs. NMOSD |
CRION vs. MS |
NMOSD vs. MS |
Statistical test |
|
Sex |
0.186 |
1.000 |
0.207 |
Fisher's exact test |
|
First unilateral or bilateral relapse |
0.269 |
1.000 |
0.202 |
Fisher's exact test |
|
Age at onset |
0.662 |
0.539 |
0.710 |
Mann-Whitney test |
|
Total number of relapses |
0.008*
|
0.226 |
0.298 |
Mann-Whitney test |
|
Number of relapses before index event |
0.208 |
0.167 |
0.548 |
Mann-Whitney test |
|
Number of relapses after index event |
|
|
0.356 |
Mann-Whitney test |
|
Unilateral VA ≤20/200 |
0.209 |
0.141 |
0.007*
|
Fisher's exact test |
|
Bilateral VA ≤20/200 |
0.706 |
0.354 |
0.177 |
Fisher's exact test |
|
Initial VFSS |
0.525 |
0.050*
|
0.002*
|
Mann-Whitney test |
|
Final VFSS |
0.448 |
0.035*
|
0.011*
|
Mann-Whitney test |
|
aRR |
0.884 |
0.930 |
0.967 |
Mann-Whitney test |
|
Disease duration |
0.159 |
0.599 |
0.248 |
Mann-Whitney test |
|
Time to first medical appointment |
0.369 |
0.861 |
0.322 |
Mann-Whitney test |
|
Time to diagnosis |
|
|
0.039*
|
Mann-Whitney test |
|
Follow-up period |
0.145 |
0.726 |
0.567 |
Mann-Whitney test |
|
Time to involvement of both eyes |
0.559 |
0.452 |
0.534 |
Mann-Whitney test |
|
Time to index event |
|
|
0.512 |
Mann-Whitney test |
|
Time between first and second relapses |
0.076 |
0.537 |
0.149 |
Mann-Whitney test |

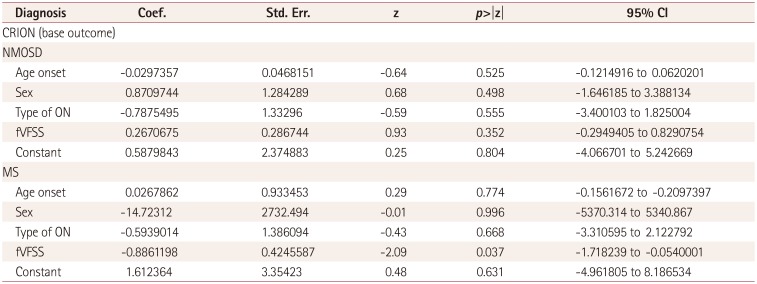
In the multinomial regression univariate analysis, both the initial and final VFSSs were associated with the final diagnosis, with lower scores increasing the probability of an MS diagnosis (
Supplementary Table 1 and
2 in the online-only Data Supplement). In the multivariate analysis, the model with age, sex, type of ON, and final VFSS showed that the final VFSS remained associated with the final diagnosis of MS, contributing 27% of the variance in this final diagnosis (
Table 3).
Table 3
Multinomial logistic regression of predictive factors for the final diagnosis
|
Diagnosis |
Coef. |
Std. Err. |
z |
p>|z| |
95% CI |
|
CRION (base outcome) |
|
|
|
|
|
|
NMOSD |
|
|
|
|
|
|
Age onset |
−0.0297357 |
0.0468151 |
−0.64 |
0.525 |
−0.1214916 to 0.0620201 |
|
Sex |
0.8709744 |
1.284289 |
0.68 |
0.498 |
−1.646185 to 3.388134 |
|
Type of ON |
−0.7875495 |
1.3329600 |
−0.59 |
0.555 |
−3.400103 to 1.825004 |
|
fVFSS |
0.2670675 |
0.286744 |
0.93 |
0.352 |
−0.2949405 to 0.8290754 |
|
Constant |
0.5879843 |
2.3748830 |
0.25 |
0.804 |
−4.066701 to 5.242669 |
|
MS |
|
|
|
|
|
|
Age onset |
0.0267862 |
0.9334530 |
0.29 |
0.774 |
−0.1561672 to −0.2097397 |
|
Sex |
14.7231200 |
2732.494000 |
−0.01 |
0.996 |
−5370.314 to 5340.867 |
|
Type of ON |
−0.5939014 |
1.3860940 |
−0.43 |
0.668 |
−3.310595 to 2.122792 |
|
fVFSS |
0.8861198 |
0.424559 |
−2.09 |
0.037 |
−1.718239 to −0.0540001 |
|
Constant |
1.612364 |
3.35423 |
0.48 |
0.631 |
−4.961805 to 8.186534 |

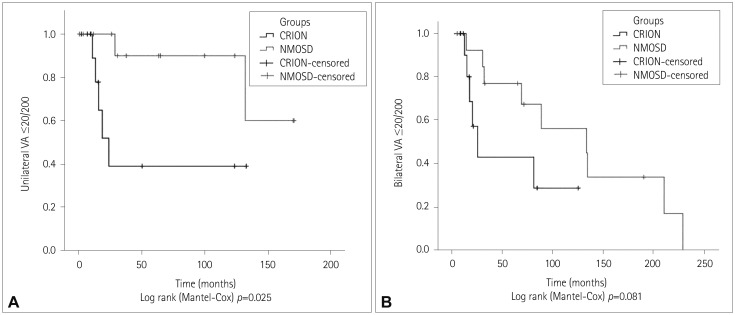
The times to reach VA ≤20/200 in the worst eye and in both eyes were compared between NMOSD and CRION (
Fig. 3). Patients with CRION reached unilateral VA ≤20/200 faster than patients with NMOSD (
p=0.025) (
Table 2). However, the time to reach bilateral VA ≤20/200 (
p=0.081) and to reach the same level of disability did not differ between patients who were positive and negative for anti-AQP-4 antibody (data not shown).
Fig. 3
A: Kaplan-Meier curve for unilateral VA ≤20/200 according to the diagnosis. B: Kaplan-Meier curve for bilateral VA ≤20/200 according to the diagnosis. p<0.05, statistically significant difference between the groups. CRION: chronic relapsing inflammatory optic neuropathy, NMOSD: neuromyelitis optica spectrum disorders, VA: visual acuity.

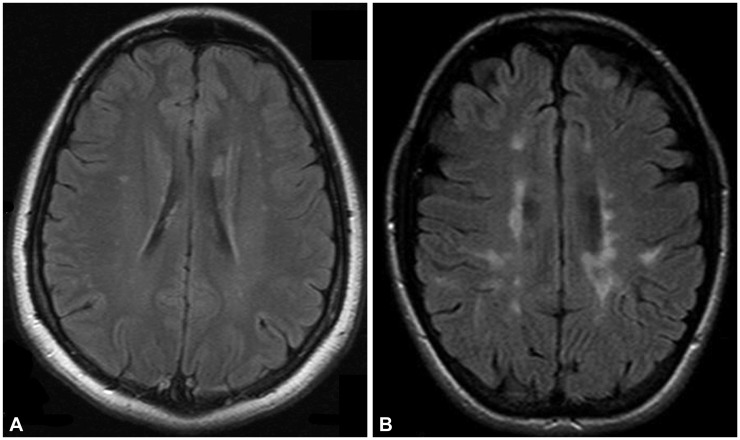
Brain white-matter abnormalities were observed in 23.1% (3/13), 42.9% (6/14), and 100% (6/6) of the patients with final diagnoses of CRION, NMOSD, and MS, respectively. Although patients with CRION and NMOSD had nonspecific brain white-matter abnormalities, patients with a final diagnosis of MS met the McDonald 2010 diagnostic criteria.
14 The optic-nerve images were clearly abnormal in 23.1% (3/13) of patients with CRION and in 14.3% (2/14) of patients with NMOSD.
Fig. 4 shows two illustrative cases of CRION and MS.
Fig. 4
Comparison between brain white-matter abnormalities in patients with final diagnoses of chronic relapsing inflammatory optic neuropathy (A) and multiple sclerosis (B).

The anti-AQP-4 antibody test was performed in 21 (63.6%) patients: 12 with a final diagnosis of NMOSD and 9 with CRION. All nine anti-AQP-4-antibody-positive patients—according the new criteria—were included in the NMOSD group. Seven of the positive patients (77.8%) developed transverse myelitis during follow-up.
Patients with NMOSD and MS received immunosuppressant or immunomodulatory treatment accordingly. Patients with CRION were treated with azathioprine and/or corticoid, similarly to NMOSD. Immunosuppressant treatment was well tolerated in this group, but its impacts on disease progression and relapse frequency were not measured.
DISCUSSION
The present study aimed to characterize the clinical, epidemiological, and radiological data of patients with relapsing ON according to the final diagnosis of CRION, NMOSD, or MS, and identify factors that can be used to predict these diseases. We studied 33 patients, comprising 13 with CRION, 14 with NMOSD, and 6 with MS.
Our CRION, NMOSD, and MS patients showed comparable aRR values. We observed a predominance of unilateral ON relapses in CRION, which differs from the initial description of 53.3% of CRION patients having bilateral opticnerve involvement from the onset.
7
The main predictor of the final diagnosis was the VFSS. ON is more severe in NMOSD and CRION, with an initial VFSS of ≥5 in 85.7% and 63.6% of patients, respectively. Only 16.7% of patients with MS reached this level of visual impairment. A particularly interesting observation was that despite showing a better VA, patients with CRION reached unilateral VA ≤20/200 faster than did patients with NMOSD.
While the final VFSS was a predictive factor in the multivariate analysis, it contributed only 27% of the variance in the final diagnosis of MS. Such a modest impact could be explained by the small number of patients in the model or by the existence of other factors that were not included in the analysis due to the retrospective design of our study.
The presence of anti-AQP-4 antibody was associated with worse VA and a greater risk of conversion to NMOSD. Spinal-cord relapse occurred in 77.8% of the patients who were positive for anti-AQP-4 antibody. A recent study involving a predominantly Caucasian population found that 50% of anti-AQP-4-antibody-positive patients would presente a spinal-cord relapse within 2 years of follow-up.
8 NMOSD is more prevalent in non-Caucasians, and up to 22% of Brazilian patients with a demyelinating disease reportedly have NMOSD.
17 Thus, the higher frequency of transverse myelitis in our study could be explained by the higher prevalence of NMOSD in Brazil. On the other hand, two of the patients that remained as relapsing ON (13.3%) were positive for anti-AQP-4 antibody, which is similar to previous descriptions.
21115
While white-matter abnormalities were seen in NMOSD, CRION, and MS, as expected, only patients with MS had lesions that fulfilled the mandatory dissemination in space according to the McDonald 2010 criteria.
1418 However, it is important to highlight that almost one-quarter of patients with CRION may present with nonspecific brain lesions on MRI. Thus, MRI findings should not prevent a diagnosis of CRION, when appropriate.
Our study was subject to several limitations. It performed a retrospective analysis of reviewed medical records, and hence there will inevitably be biases due to how the information was recorded. In addition, the small number of patients prevented us from drawing definitive conclusions, despite our findings being consistent with those already reported in the literature.
It has recently been shown that NMOSD patients who are negative for anti-AQP-4 antibody and some with isolated ON may be positive for antibodies against myelin oligodendrocyte glycoprotein (anti-MOG antibody). Most series are consistent with anti-MOG antibody being present in up to 25% of anti-AQP-4-antibody-negative patients, and being preferentially associated with acute disseminated encephalomyelitis, transverse myelitis, and severe relapsing ON.
192021 There is no anti-MOG antibody assay commercially available in Brazil for clinical use, and so our patients with relapsing ON were not tested, which we accept is a major limitation of our study.
Larger studies with a prospective design are necessary to better understand the phenotypic characteristics of patients with relapsing ON and thereby improve their care. Meanwhile, due to the relapse severity and patients with CRION responding well to immunosuppression, we suggest that relapsing ON—when atypical for MS—should be treated early to avoid new relapses and permanent disability.
Acknowledgements
Authors would like to thank Marcos Hideki Idagawa MD for the help in obtaining magnetic resonance images from the hospital's system.
References
1. Optic Neuritis Study Group. Visual function 15 years after optic neuritis: a final follow-up report from the optic neuritis treatment trial. Ophthalmology. 2008; 115:1079–1082.e5. PMID:
17976727.
2. Jarius S, Frederikson J, Waters P, Paul F, Akman-Demir G, Marignier R, et al. Frequency and prognostic impact of antibodies to aquaporin-4 in patients with optic neuritis. J Neurol Sci. 2010; 298:158–162. PMID:
20850793.

3. Hickman SJ, Dalton CM, Miller DH, Plant GT. Management of acute optic neuritis. Lancet. 2002; 360:1953–1962. PMID:
12493277.

4. Beck RW, Cleary PA, Anderson MM Jr, Keltner JL, Shults WT, Kaufman DI, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992; 326:581–588. PMID:
1734247.
5. Abou Zeid N, Bhatti MT. Acute inflammatory demyelinating optic neuritis: evidence-based visual and neurological considerations. Neurologist. 2008; 14:207–223. PMID:
18617847.
6. Benoilid A, Tilikete C, Collongues N, Arndt C, Vighetto A, Vignal C, et al. Relapsing optic neuritis: a multicentre study of 62 patients. Mult Scler. 2014; 20:848–853. PMID:
24177207.

7. Kidd D, Burton B, Plant GT, Graham EM. Chronic relapsing inflammatory optic neuropathy (CRION). Brain. 2003; 126:276–284. PMID:
12538397.

8. Matiello M, Lennon VA, Jacob A, Pittock SJ, Lucchinetti CF, Wingerchuk DM, et al. NMO-IgG predicts the outcome of recurrent optic neuritis. Neurology. 2008; 70:2197–2200. PMID:
18434643.

9. de Seze J, Arndt C, Jeanjean L, Zephir H, Blanc F, Labauge P, et al. Relapsing inflammatory optic neuritis: is it neuromyelitis optica? Neurology. 2008; 70:2075–2076. PMID:
18505981.
10. Marignier R, De Sèze J, Vukusic S, Durand-Dubief F, Zéphir H, Vermersch P, et al. NMO-IgG and Devic's neuromyelitis optica: a French experience. Mult Scler. 2008; 14:440–445. PMID:
18208892.

11. Petzold A, Pittock S, Lennon V, Maggiore C, Weinshenker BG, Plant GT. Neuromyelitis optica-IgG (aquaporin-4) autoantibodies in immune mediated optic neuritis. J Neurol Neurosurg Psychiatry. 2010; 81:109–111. PMID:
20019228.

12. Petzold A, Plant GT. Chronic relapsing inflammatory optic neuropathy: a systematic review of 122 cases reported. J Neurol. 2014; 261:17–26. PMID:
23700317.

13. Collongues N, Marignier R, Zéphir H, Blanc F, Vukusic S, Outteryck O, et al. High-risk syndrome for neuromyelitis optica: a descriptive and comparative study. Mult Scler. 2011; 17:720–724. PMID:
21239412.

14. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69:292–302. PMID:
21387374.

15. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015; 85:177–189. PMID:
26092914.

16. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983; 33:1444–1452. PMID:
6685237.

17. Papais-Alvarenga RM, Carellos SC, Alvarenga MP, Holander C, Bichara RP, Thuler LC. Clinical course of optic neuritis in patients with relapsing neuromyelitis optica. Arch Ophthalmol. 2008; 126:12–16. PMID:
18195212.

18. Swanton JK, Fernando KT, Dalton CM, Miszkiel KA, Altmann DR, Plant GT, et al. Early MRI in optic neuritis: the risk for clinically definite multiple sclerosis. Mult Scler. 2010; 16:156–165. PMID:
20086028.

19. Biotti D, Bonneville F, Tournaire E, Ayrignac X, Dallière CC, Mahieu L, et al. Optic neuritis in patients with anti-MOG antibodies spectrum disorder: MRI and clinical features from a large multicentric cohort in France. J Neurol. 2017; 264:2173–2175. PMID:
28914353.

20. Cobo-Calvo Á, Ruiz A, D'Indy H, Poulat AL, Carneiro M, Philippe N, et al. MOG antibody-related disorders: common features and uncommon presentations. J Neurol. 2017; 264:1945–1955. PMID:
28770374.

21. Hennes EM, Baumann M, Schanda K, Anlar B, Bajer-Kornek B, Blaschek A, et al. Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology. 2017; 89:900–908. PMID:
28768844.

Supplementary Materials
Supplementary Table 1
Multinomial logistic regression of predictive factors of the final diagnosis: univariate analysis of the iVFSS
jcn-14-351-s001.pdf
Supplementary Table 2
Multinomial logistic regression of predictive factors of the final diagnosis: univariate analysis of the fVFSS
jcn-14-351-s002.pdf










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download