INTRODUCTION
METHODS
Statistical analyses
RESULTS
Demographics
Table 1
Demographic variables of the 81 patients
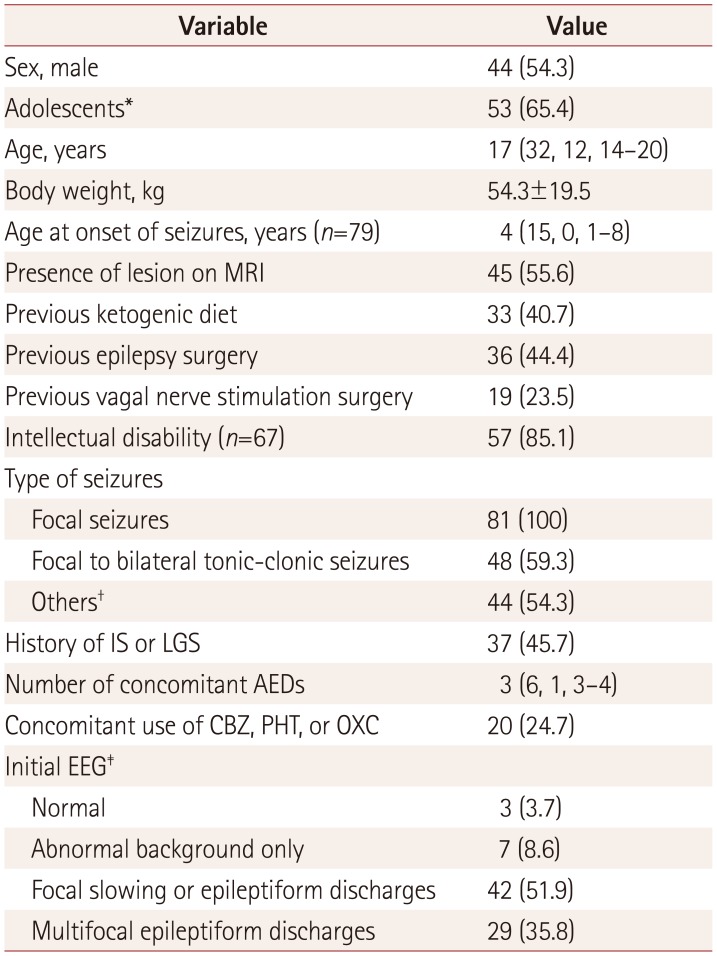
Data are median (maximum, minimum, interquartile range), mean±standard-deviation, or n (%) values.
*Up to 18-years-old, †Epileptic spasm (n=11), atypical absence (n=10), drop attacks (n=8), eyelid myoclonus (n=4), or tonic seizure (n=24); 15 patients had multiple seizure types, ‡EEG immediately before administering perampanel.
AED: antiepileptic drug, CBZ: carbamazepine, IS: infantile spasms, LGS: Lennox-Gastaut syndrome, OXC: oxcarbazepine, PHT: phenytoin.
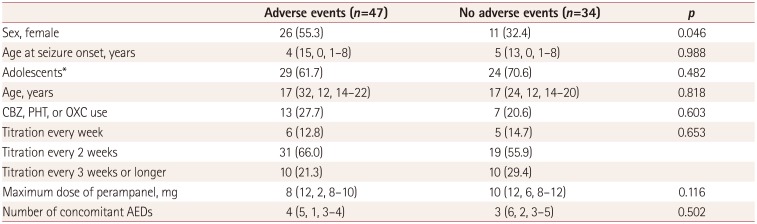
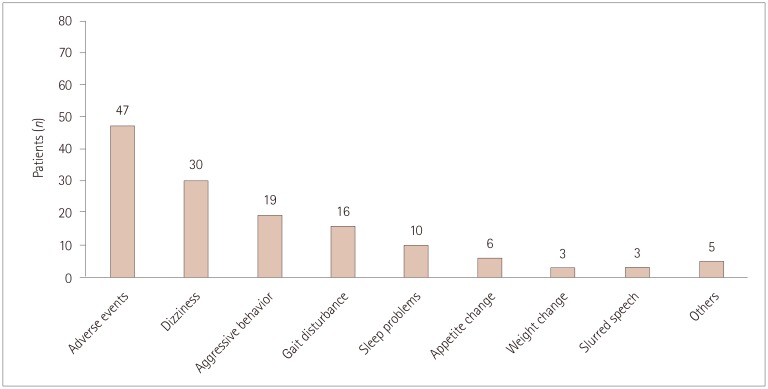
Adverse events and related factors
Table 2
Comparison of fast titration (2-mg increments at intervals of 1 to 2 weeks) and slow titration (2-mg increments at intervals of 3 weeks or longer)
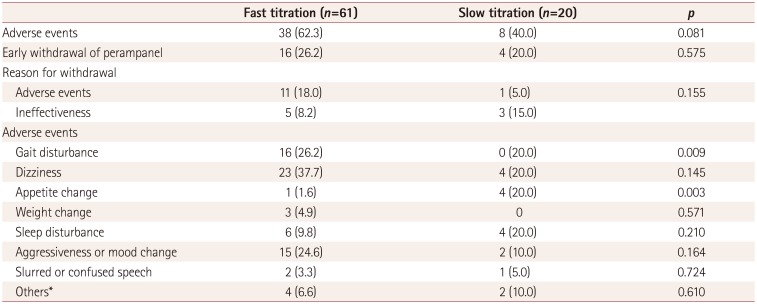
Table 3
Comparison between patients who experienced and did not experience adverse events
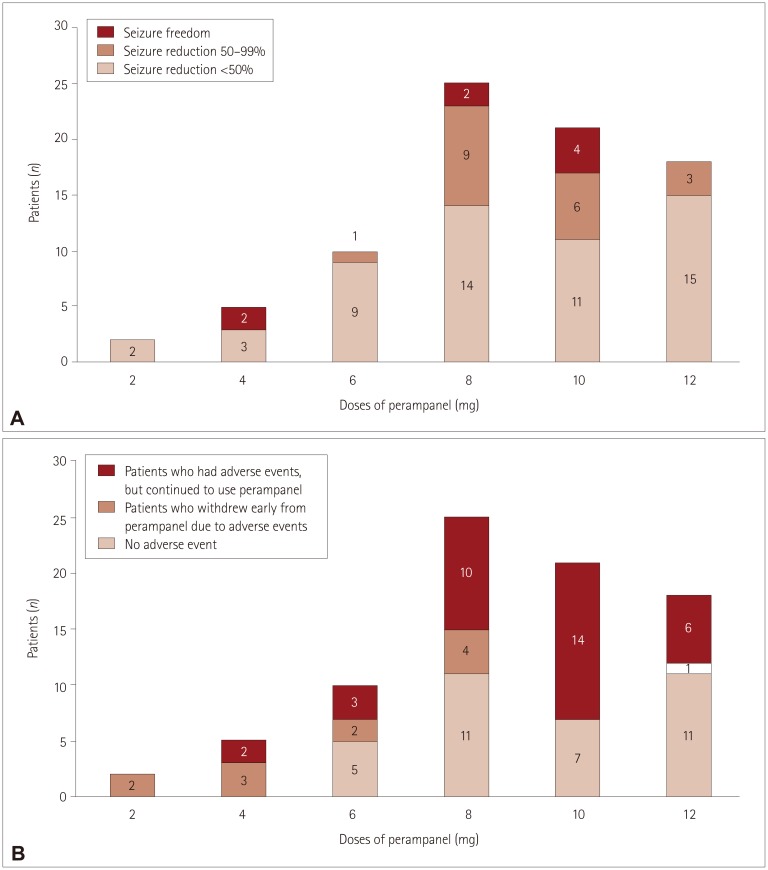 | Fig. 2Three-month seizure outcomes and occurrence of adverse events for different maximum doses of perampanel. A: The maximum doses were 2 and 4 mg in 7 patients. Two patients achieved seizure freedom when taking perampanel at the relatively low dose of 4 mg. The maximum doses were 6, 8, and 10 mg in more than two-thirds (56 of 81 patients) of patients. The rates of responders and seizure freedom were high for doses between 6 and 10 mg. The perampanel dose was increased to 12 mg when their seizures persisted, and no serious adverse events occurred. However, the rates of responders and seizure freedom were significantly low at a dose of 12 mg. B: Five patients stopped taking perampanel at low doses (e.g., 2 or 4 mg) due to adverse events. Only one patient stopped taking perampanel at 12 mg. Two patients who stopped at a dose of 2 mg experienced severe dizziness, and three patients who stopped at a dose of 4 mg reported experiencing multiple side effects including gait disturbance, dizziness, and aggressive behavior. |
Efficacy
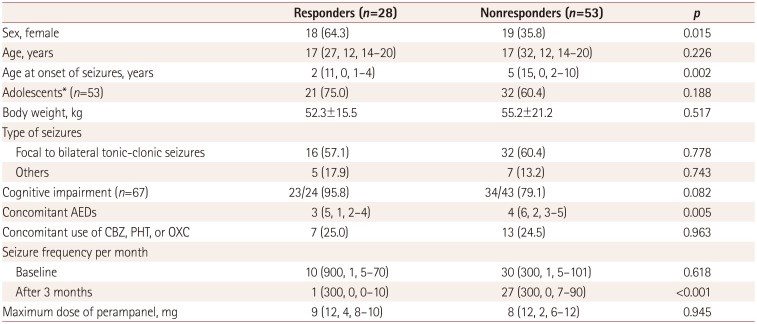
Table 4
Comparison of responders (reduction in seizure frequency of ≥50%) and nonresponders




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download