INTRODUCTION
Orthostatic hypotension (OH) is a common condition that occurs when a patient shows a reduction in systolic blood pressure (BP) of at least 20 mm Hg or in diastolic BP of at least 10 mm Hg within 3 minutes of standing or after a head-up tilt (HUT) to at least 60 degrees on a tilt table.
12345 The severity of OH symptoms can vary substantially between patients, with some patients failing to display even minor symptoms during the HUT test in spite of showing the characteristic BP decrease.
6 Unfortunately, it is difficult to predict either the presence or the severity of clinical symptoms for any specific patient.
OH is known to be caused by adrenergic sympathetic dysfunction.
567 Although OH is defined solely based on a minimum threshold for a change in BP, the magnitude of BP decreases and the patterns of BP changes during the HUT test are highly variable. Some patients show rapid recovery in BP after tilting while others show a continuous decrease over time for as long as the tilted position is maintained. These variations could be related to the ability of the autonomic nervous system to compensate for changes in BP. Two previous studies have measured the patterns of BP changes in different OH patients.
89 However, both of these studies mainly focused on supine hypertension with OH
9 or otherwise were limited to the minimum duration of HUT necessary to detect OH,
8 without focusing on the clinical significance of these patterns of BP changes. Additionally, the authors applied a HUT protocol that involved only 5 minutes of tilting, which might be insufficient to observe meaningful patterns in BP changes. Finally, their samples were likely to be too small to allow any significant clinical conclusions to be drawn. A recent study also investigated the patterns of BP changes in OH patients, but it targeted all types of OH including delayed OH; that is, not just classic OH alone.
10 This approach meant that the study could not provide information on the detailed pattern classification of classic OH, which constitutes the largest proportion of all types of OH.
The aim of the present study was to identify the association between the patterns of BP changes during the HUT test and symptom development during tilting, as well as to obtain other autonomic function test (AFT) results for OH patients.
DISCUSSION
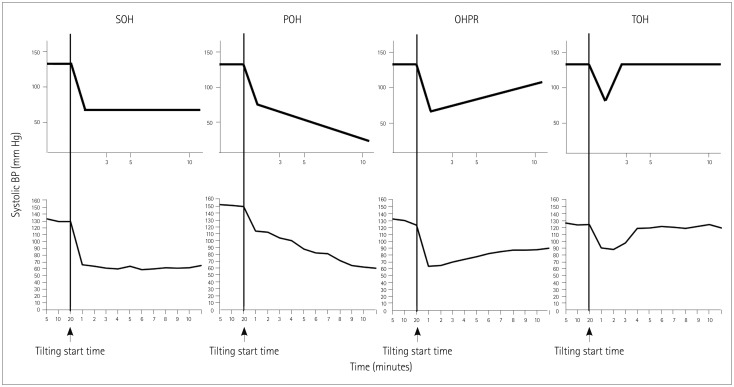
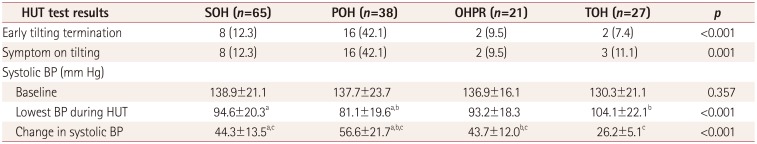
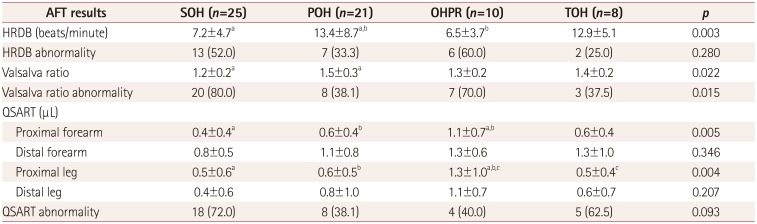
This study explored the patterns of BP changes during the HUT test and its clinical significance in OH patients. Based on the first 10 minutes after HUT, we identified four patterns of BP changes among OH patients, which we termed SOH, POH, OHPR, and TOH (as defined in the Methods section). Key measurements from the HUT test and other AFTs were found to differ significantly between these four OH groups. POH patients exhibited both the largest decrease in systolic BP after tilting and the greatest likelihood of developing symptoms resulting in early HUT termination. Additionally, patients with SOH had smaller HRDB values and Valsalva ratios compared to POH patients, and OHPR patients had greater proximal forearm and leg sweat volumes.
We observed that SOH (43.0%) was the most common pattern among the OH patients in our study, followed by POH (25.2%), TOH (17.9%), and OHPR (13.9%). To our knowledge there are two other published studies that have also evaluated OH patterns.
89 One study found five patterns of orthostatic BP response, which were described as stable OH, OH with partial recovery, OH with late recovery, POH, and OH with delayed worsening. That study investigated 66 patients with OH, and most of the patients were assigned to groups with either stable OH (48%) or POH (36%),
8 which respectively correspond to our SOH and POH groups. OH with partial recovery in the previous study was similar to SOH in our study, but those authors did not consider the transient pattern. The second study found three distinct OH patterns, which were described as SOH, POH, and TOH.
9 Most of the included 70 patients (55.7%) were categorized as SOH, which is in line with our results. However, unlike in the present study, TOH was more frequent (27.1%) than POH (17.1%). The inconsistencies between our study and each of the other two studies may be due to the application of slightly different definitions for OH patterns, as well as differences in the duration of the tilting time during HUT. We defined a new pattern, called OHPR, which exhibited a slight but incomplete recovery after the initial BP decrease. Perhaps more importantly, whereas these previous studies focused on the initial 5 minutes of tilting, we considered systolic BP changes up to 10 minutes after beginning the head tilt as part of our classification system. Because of these differences, patients in our OHPR category might have been categorized as TOH when applying a HUT test that only considered the period up to 5 minutes after tilting the head.
The patterns of BP changes for different OH patients can provide information for predicting the HUT outcome.
89 Similar to previous studies,
89 our patients with POH showed the highest proportion of cases (42.1%, 16/38) of early termination of tilting and symptom development during the HUT test. In addition, the POH patients exhibited the greatest decrease in systolic BP during tilting, suggesting a greater impairment of adrenergic sympathetic function. In support of this notion, two previous studies using the Valsalva maneuver demonstrated that higher adrenergic scores in the composite autonomic severity score occurred in OH patients with a progressive pattern.
89 These dramatic adrenergic deficits imply the presence of severe impairment of the compensatory reflex in those patients, and that this contributes to their poorer HUT outcomes.
Conversely, even though the supine-to-tilt systolic BP changes were greater in patients with SOH or OHPR than in TOH patients, the rate of symptom development did not differ between these groups. A recent study using Model-flow measurements of total peripheral resistance and cardiac output classified OH into three categories: 1) arteriolar OH, characterized by a predominant decrease in total peripheral resistance, 2) venular OH, characterized by a predominant decrease in cardiac output, and 3) mixed OH, characterized by decreases in both of these parameters.
16 On the one hand, patients with mixed OH showed the greatest posttilt reduction in systolic BP (median 53.3 mm Hg, range 45.59–66.76 mm Hg), and symptoms developed in 46% of these patients. On the other hand, the decrease in systolic BP was smaller in patients with venular OH (median 31.9 mm Hg, range 28.12–40.78 mm Hg) than in patients with arteriolar OH (median 42.5 mm Hg, range 30.65–46.93 mm Hg) and mixed OH, and yet the proportion of patients reporting symptoms was higher or similar in venular OH (47%) than in arteriolar (33%) and mixed (46%) OH. Together these findings suggest that, in addition to the magnitude of the BP decrease, other factors are involved in the development of symptoms experienced by OH patients during the HUT test. A different main determinant for the decrease in BP in each OH category could affect symptom occurrence. One previous study found that the main determinant of the BP decrease leading to symptoms was a significant reduction in cardiac output due to reduced venous return.
16 Those authors also found that the reduction in cardiac output was significantly greater in symptomatic OH patients.
In our study we found that patients with SOH had smaller HRDB values and Valsalva ratios compared to POH patients, suggesting a significant decrease in cardiovagal activity. A previous study found that the adrenergic score of the composite autonomic severity score tended to be higher in patients with POH than in patients with SOH.
8 However, there was no significant difference in cardiovagal, sudomotor, or total scores on the composite autonomic severity score between these two OH groups. In another study, patients with POH also had higher scores on the adrenergic subscale of the composite autonomic severity score compared to patients presenting other OH patterns.
9 However, those authors did not report the cardiovagal, sudomotor, or total scores on the composite autonomic severity score for each OH pattern. Therefore, the presence of greater adrenergic dysfunction in POH than in SOH has been confirmed in previous studies and is consistent with our study. However, the differences of cardiovagal and sudomotor functions between POH and SOH are still difficult to determine based on previous results.
Our study is meaningful in showing that cardiovagal dysfunction might be greater in SOH than in POH. In addition, reduced heart-rate variability has been shown to be associated with increased risks of cardiovascular morbidity and mortality. Thus, the SOH pattern may be associated with an increased risk of cardiovascular morbidity and mortality. Meanwhile, patients with OHPR exhibited the highest proximal forearm and leg sweat volumes. Our QSART findings show that the increased sweat volume indicates a relatively preserved cholinergic sympathetic function for OHPR patients. The parameters measured in OHPR patients for both the HUT and other AFTs are similar to those measured in SOH patients, with the exception of the QSART findings. This suggests that OHPR is a mild form of SOH in which cholinergic sympathetic function is preserved. Our findings therefore suggest that the severity and type of autonomic system dysfunction can be predicted by analyzing the patterns of BP changes during the HUT test in patients with OH. In other words, the different types of OH might be related to the different mechanisms that underlie autonomic dysfunction.
Prior studies have classified OH patterns based on HUT results obtained only during the initial 5 minutes of the test.
89 However, we found that 5 minutes of orthostatic stress may be inadequate for defining the most meaningful patterns of BP changes. A significant proportion of the cases in our study (32%, 26/81) exhibited SOH patterns during the first 5 minutes, but then their BP changed significantly over the following 5 minutes. Of the 26 patients in our study allocated to the SOH group, 16 were redesignated as POH and 9 were redesignated as OHPR. In addition, 78 patients excluded from our final analysis based on fluctuating BP changes over 10 minutes would have been classifiable under the 5-minute criterion. Based on these findings, we suggest that a 5-minute HUT test does not yield sufficient data for characterizing the elaborate patterns of OH, even if 5 minutes is sufficient for diagnosing OH. Therefore, the orthostatic stress beyond 5 minutes should be measured when assessing clinically relevant patterns of BP changes among OH patients.
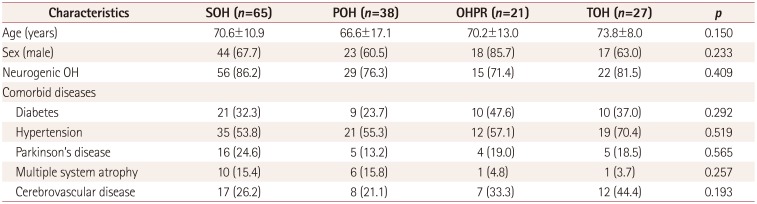
There are some potential limitations to our study that should be considered. Our data contained insufficient clinical information regarding medications that can potentially affect autonomic function due to the retrospective study design. To minimize the possible effects of medications, all subjects discontinued taking drugs for at least 24 hours prior to performing AFTs. Furthermore, our study included patients with various comorbid conditions that can contribute to OH. Although these comorbid conditions did not differ among the four OH groups in the present study, we could not exclude a possible influence of each OH pattern on the AFT results. However, we analyzed a relatively large amount of data compared to previous studies, and so this study improves the likelihood that such information could be applied in clinical evaluations of patients with OH. The analysis of other AFT results in OH patterns was also limited by the amount of missing data, which reduced the effective total sample size for this analysis to only 64 patients.
We performed manual BP measurements every minute rather than continuously monitoring the beat-to-beat BP. This use of intermittent BP monitoring can make differentiating the patterns of BP changes during a HUT artificial and unclear. In addition, we might have missed brief OH episodes such as initial OH, which constituted 30% of the total number of OH cases in a previous study that employed continuous BP monitoring.
10 However, continuous noninvasive beat-to-beat BP monitoring performed using a finger plethysmographic device (e.g., Finapres) also has some limitations. The Finapres device reconstructs the brachial artery pressure from the measured finger arterial pressure using generalized waveform filtering and level correction. The arterial pressure waveform normally changes gradually from the brachial artery to the finger arteries as a result of the narrowing of the arteries, which can affect the accuracy of assessments of the arterial pressure. This physiologic phenomenon causes differences between the brachial and finger artery BPs. Furthermore, when peripheral vasoconstriction or poor vascular circulation is present, the BP measured in a finger is not necessarily representative of the brachial BP. There is a recent report of artificial drift in the plethysmographic finger BP being misinterpreted as OH.
17 In particular, those authors showed that an artificial transient decrease in the plethysmographic finger BP could occur at the beginning of tilting. This can be fatal to evaluate the initial OH that occurs within 15 seconds of standing.
2 Furthermore, passive tilting is not adequate for evaluating the initial OH; the condition can only be documented by employing active standing with continuous BP monitoring.
218 While the lack of continuous BP monitoring using the Finapres device is a limitation of our study, this device is not always available for use in actual clinical practice. The Finapres device is not essential for performing the HUT test, and so our methods for the pattern analysis of OH using manual sphygmomanometer BP recordings can be more useful to clinicians in actual clinical practice.
Finally, caution should be applied when interpreting Valsalva ratio results as cardiovagal dysfunction when there is no information on simultaneous BP changes. However, in our study the Valsalva ratio decreased with the HRDB in SOH. The HRDB is a more sensitive and powerful test than the Valsalva ratio since both efferent and afferent pathways are vagal. Therefore, our results suggest that the cardiovagal dysfunction is greater in SOH than in POH regardless of the results for the Valsalva ratio.
These results show that not only is the HUT test useful for diagnosing OH, but also suggest the clinical significance of this test in differentiating the patterns of BP changes over a 10-minute test and associating those differences with the symptoms presented by those patients as well as their severity. OH patients present widely varying patterns of symptoms in HUT tests, and each pattern may have a different clinical significance. Therefore, our approach can provide additional valuable information for managing the disease in individual OH patients.
In conclusion, certain clinical measurements (including AFT results) show meaningful differences between different OH patient groups defined based on the patterns of systolic BP changes during head-up tilting in the HUT test. These differences might reflect different underlying pathophysiologic mechanisms, and so different management strategies could be applied to specific OH patients in order to improve the likelihood of successful outcomes.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download