Dear Editor:
Distinguishing acral lentiginous melanoma (ALM) from acral nevi during diagnosis is challenging, but diagnostic accuracy is important because the therapeutic strategies for these diseases differ123456. Insulin-like growth factor-II mRNA-binding protein 3 (IMP-3) is expressed in some malignancies, including malignant melanoma (MM). Therefore, we investigated IMP-3 expression in a spectrum of melanocytic lesions, including acral nevi, dysplastic nevi, Spitz nevi, ALM, and metastatic MMs in Korean patients. We assessed the utility of IMP-3 as a diagnostic and invasiveness biomarker for distinguishing ALM from benign nevi in acral lesions.
Biopsied and surgically resected melanocytic tumor samples obtained between January 2001 and October 2014 from 239 subjects were retrieved from the Departments of Dermatology and Pathology at Dong-A University Hospital. The samples included benign melanocytic nevi (n=24) not located in acral areas, dysplastic nevi (n=16), Spitz nevi (n=18), acral nevi (n=75), in situ MM (n=20), primary ALM (n=72), and metastatic MM (n=14). All cases were diagnosed by a dermatopathologist using standard criteria. Hematoxylin and eosin-stained slides were examined to confirm the diagnosis, and representative sections were selected for immunohistochemistry (IHC). The study was approved by the Institutional Review Board of Dong-A University Hospital (IRB no. DAUH 18-084).
IHC was performed on 5-µm formalin-fixed, paraffin-embedded tissue sections using a mouse monoclonal antibody against IMP-3 (clone 69.1; Dako, Carpinteria, CA, USA). Tissue sections were deparaffinized using established procedures78. Slides were mounted using an aqueous medium and viewed using a Nikon Eclipse E600 light microscope (Nikon, Tokyo, Japan) equipped with an Olympus DP70 digital camera (Olympus Corp., Tokyo, Japan). For the negative control, the primary antibody was replaced with 5% fetal bovine serum.
IMP-3 expression was independently evaluated by two dermatopathologists. The proportion of tumor cells positive for IMP-3 was recorded as diffuse positive (>50%), focal or heterogeneous (10%~50%), and trace (<10%). Cytoplasmic staining intensity was recorded as negative, weak, moderate, or strong according to previous reports78. When scores differed between investigators, the slides were re-evaluated under a multi-headed microscope until a consensus was reached.
Statistical analyses were performed using Statistical Package for Social Sciences (version 18.00; SPSS Inc., Chicago, IL, USA). Categorical variables were analyzed using Pearson's chi-squared test and Fisher's exact test, and continuous variables were analyzed using a one-way analysis of variance and Student's t-test. p<0.05 was considered significant.
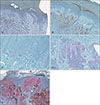
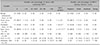
IMP-3 expression results for the 239 melanocytic neoplasm samples are summarized in Table 1. IMP-3 positivity was observed in less than 10% of lesion cells of one acral nevus, one dysplastic nevus, and two Spitz nevi. Of 20 in situ MM, IMP-3 was detected in <10%. Expression was detected in 10%~50% of tumor cells in eight (40%) and four (20%) MM samples. IMP-3-positive cells were arranged as isolated single cells or rare small aggregates (Fig. 1). Seventy of 72 (97.2%) ALMs expressed IMP-3. Expression was observed in <10% and ≥10% of tumor cells in 17 (23.6%) and 54 (76.4%) ALM samples, respectively. IMP-3 expression in ALM differed significantly from that in benign, acral, dysplastic, or Spitz nevi (p<0.001 individually and in combination) (Fig. 1). Eight (40%), eight (40%), two (20%), and two (20%) in situ MM samples exhibited negative, weak, moderate, and strong staining, respectively (Table 1). For ALMs, 14 (19.4%), 31 (43.1%), and 25 (34.7%) samples were scored as weak, strong, and moderate, respectively. One acral, one dysplastic, and one Spitz nevus exhibited weak expression and a single Spitz nevus exhibited moderate expression.
IMP-3 expression differed significantly between ALMs and benign melanocytic neoplasms with or without dysplastic features. Most ALMs (73.6%) expressed IMP-3 in ≥10% of tumor cells, and most ALMs (77.8%) presented moderate or strong IMP-3 expression. Conversely, acral nevus samples showed no or little IMP-3 expression. These results are consistent with those of previous studies indicating that a melanoma diagnosis, rather than a melanocytic nevi diagnosis is appropriate when IMP-3 is detected. Pryor et al.7 demonstrated that IMP-3 is expressed in malignant melanoma, but not in benign nevi, even when dysplastic features are present. Additionally, Yu et al.8 showed that IMP-3 expression differs significantly between non-desmoplastic melanomas and benign, dysplastic, or Spitz nevi. IMP-3 expression differences between acral nevi and ALM are helpful for distinguishing between the two. However, 80% of in situ MM were negative or weakly positive for IMP-3, and in cases with no IMP-3 expression, in situ MM cannot be ruled out. Therefore, strong IMP-3 expression favors a melanoma diagnosis when the differential diagnosis of a melanocytic tumor includes acral nevus, dysplastic nevus, and Spitz nevus. We suggest that IMP-3 staining is a useful invasiveness marker for ALM differentiation with acral nevi.
IMP3 overexpression might contribute to local invasion of ALM. Differences in staining intensity in ALM are thought to be related to tumor depth. Sheen et al.910 reported that IMP-3 expression is correlated with thick and high-grade tumors and predicts poorer overall, melanoma-specific, recurrence-free, and distant metastasis-free survivals. Patients with a tumor thickness of <4.0 mm and positive IMP-3 expression had a significantly worse melanoma-specific survival than that of patients without IMP-3 expression. The association between IMP3 overexpression and worse disease-specific survival also suggested that IMP3 has an oncogenic role.
IMP-3 may regulate the stability of high-motility group AT-hook-2 (HMGA2) mRNA, which encodes a non-histone chromatin-binding protein associated with melanoma invasion10. IMP-3 may interact with HMGA2 to activate migration and invasion pathways, which could induce the highly malignant phenotype observed in clinical specimens. Further targets should be investigated to elucidate the mechanism by which IMP-3 promotes MM formation and invasion.
Our study had some limitations related to its retrospective design. First, a single ethnic group was considered. Second, the Spitz and dysplastic nevi sample sizes were small. Third, our IHC staining results were interpreted subjectively, based on a non-standardized measure of intensity. Lastly, although our results suggest that IMP-3 is an invasive marker in melanoma, long-term survival data were not evaluated. Therefore, large prospective studies may be useful to identify additional predictive features among Caucasian, African, and Asian patients with ALM and other benign melanocytic nevi.
Figures and Tables
Fig. 1
Comparison of acral nevus with early acral lentiginous malignant melanoma. (A) Acral nevus, negative (B) acral nevus, weak (C) ALM, weak (D) ALM, moderate, and (E) ALM, strong (H&E, ×200). ALM: acral lentiginous melanoma.
References
1. Chang JW, Yeh KY, Wang CH, Yang TS, Chiang HF, Wei FC, et al. Malignant melanoma in Taiwan: a prognostic study of 181 cases. Melanoma Res. 2004; 14:537–541.

2. Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986–2005. Arch Dermatol. 2009; 145:427–434.
3. Ito T, Moroi Y, Oba J, Nakahara T, Takeuchi S, Uchi H, et al. The prognostic value of a reverse transcriptase-PCR assay of sentinel lymph node biopsy for patients with cutaneous melanoma: a single-center analysis in Japan. Melanoma Res. 2012; 22:38–44.

4. Phan A, Touzet S, Dalle S, Ronger-Savlé S, Balme B, Thomas L. Acral lentiginous melanoma: a clinicoprognostic study of 126 cases. Br J Dermatol. 2006; 155:561–569.

5. Krementz ET, Feed RJ, Coleman WP 3rd, Sutherland CM, Carter RD, Campbell M. Acral lentiginous melanoma. A clinicopathologic entity. Ann Surg. 1982; 195:632–645.
6. Slingluff CL Jr, Vollmer R, Seigler HF. Acral melanoma: a review of 185 patients with identification of prognostic variables. J Surg Oncol. 1990; 45:91–98.

7. Pryor JG, Bourne PA, Yang Q, Spaulding BO, Scott GA, Xu H. IMP-3 is a novel progression marker in malignant melanoma. Mod Pathol. 2008; 21:431–437.

8. Yu L, Xu H, Wasco MJ, Bourne PA, Ma L. IMP-3 expression in melanocytic lesions. J Cutan Pathol. 2010; 37:316–322.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download