This article has been
cited by other articles in ScienceCentral.
Dear Editor:
Psoriasis is a chronic inflammatory skin disease in which interleukin (IL)-17-producing T helper (Th) 17 cells play a crucial role
1. Topical application of the toll-like receptor 7 agonist imiquimod (IMQ) on the ear and/or back skin of mice is a widely used IL-17-dependent model of psoriasis-like skin inflammation
2. The transcription factor retinoid acid-related orphan receptor γt (RORγt) is required for IL-17 production by Th17 cells
3. IMQ-induced skin inflammation is reduced both in RORγt-deficient mice and upon pharmacological inhibition of RORγt activity with novel small molecule selective RORγt inhibitors, indicating that RORγt is crucial for IMQ-induced psoriasis-like skin inflammation in mice and suggesting a potential role for RORγt inhibition for treatment of psoriasis
456.
The cardiac glycoside digoxin, which has been used for centuries to treat congestive heart failure and atrial fibrillation, binds to RORγt which leads to selective suppression of Th17 cell differentiation and IL-17 production
in vitro, with therapeutic effects of digoxin demonstrated in mouse models of IL-17-dependent disease, e.g., experimental autoimmune encephalomyelitis and atherosclerosis, respectively
78. To our knowledge, the effects of digoxin in models of psoriasis have not been reported and the aim of the current study was to investigate whether digoxin affects development of IMQ-induced psoriasis-like ear and back skin lesions in mice.
A detailed description of study materials and methods can be found in
Supplementary File 1. In brief, at 8 weeks of age, female C57Bl/6J mice received topical Aldara (5% IMQ) or vehicle cream daily for 5 days (45 mg on the shaved back [1×2 cm area] and 5 mg on the right ear). All mice were treated with either intraperitoneal (i.p.) digoxin (20 µg/mouse on day 1, 10 µg/mouse on day 3, and 20 µg/mouse on days 4 and 5) or saline (control group). The digoxin dose was somewhat lower than dosages (20~40 µg/d) previously used in other Th17-dependent disease models ameliorated by digoxin
78, but we found that the mice were in marked acute distress after the first dose, wherefore treatment was avoided on day 2. The mice (n=32; 8 mice/study group) were sacrificed on the 6th day, 24 hours after the last cream application and i.p. treatment. All experiments were performed according to the principles stated in the Danish law on animal experiments and were approved by the Animal Experiment's Inspectorate, Ministry of Justice, Denmark (no. 2012-15-2934-00119). The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the European Parliament (EU directive 2010/63/EU). The ethical policy of the University of Copenhagen complies with that of the National Institutes of Health (A5846-01).
In mice receiving topical application of vehicle cream, there were no discernible skin lesions and digoxin had no significant effects on measured variables (
Fig. 1,
Table 1). Digoxin attenuated the IMQ-induced ear thickening at days 5 and 6 (day 6: 0.26 vs. 0.30 mm,
p=0.001;
Fig. 1A). Histological evaluation showed an inhibitory effect of digoxin on IMQ-induced epidermal hyperplasia in the ear (2.1 vs. 3.3 epidermal cell layers,
p<0.001;
Fig. 1B). However, digoxin treatment increased the IMQ-induced back skin thickening at day 6 (1.13 vs. 0.94 mm,
p<0.01;
Fig. 1C), and increased erythema and scaling on the back skin (sum score 6.6 vs. 5.4,
p<0.05;
Fig. 1C). Also, there was no statistically significant effect of digoxin on IMQ-induced epidermal hyperplasia in the back skin (
Fig. 1D). IMQ application had systemic inflammatory effects with induction of splenomegaly, as well as increased plasma levels of IL-17A and the acute-phase protein, serum amyloid A (SAA) (
Table 1). Surprisingly, in mice receiving topical IMQ, digoxin administration led to increased plasma concentrations of IL-17A (31.60±13.29 vs. 19.05±5.22 pg/ml,
p<0.01) whereas plasma levels of SAA, IMQ-induced splenomegaly, and body weight at study termination were not affected by digoxin (
Table 1).
Our data showed that digoxin exerted differential effects on IMQ-induced psoriasis-like skin lesions at different anatomical locations, i.e., with reduction of skin lesion formation on the ear and exacerbation of back skin lesions, respectively. Interestingly, a similar differential effect on ear and back IMQ lesions in mice has been observed after topical treatment with sphingosine-1-phosphate (a cell growth and immune modulator)
9. This may relate to differences of target tissues (thinner skin, less hair follicles, minimal subcutaneous adipose tissue, and diverse lymph drainage in ear vs. back skin), the IMQ application procedure (shaving vs. no shaving before application) and IMQ dose (5 vs. 45 mg Aldara), respectively. Also, digoxin bioavailability may differ at the two sites and future studies should include local assessment of IL-17A and RORγt expression, Th17 cell accumulation, and kinetics of radiolabelled digoxin, respectively, in addition to experiments with other RORγt inhibitors
46. We unexpectedly found increased plasma IL-17A levels which is in contrast to reduced levels reported in hyperlipidemic apoE-deficient mice treated with digoxin for 12 weeks, but the relationship between target tissue and systemic levels of IL-17A in experimental models of disease remains poorly defined
8. Importantly, off-target effects of digoxin may play a role since in addition to RORγt inhibition the agent inhibits the sodium potassium adenosine triphosphatase (Na
+/K
+ ATPase) present in all cells and mainly in the heart. Indeed, systemic treatment with novel potent selective RORγt inhibitors appear to suppress IMQ-induced skin inflammation on both the ears and back of mice, albeit that there are no reports on simultaneous effects of these RORγt inhibitors on both anatomical locations in the same animals
46. In addition, it was recently reported that development of psoriasiform lesions after topical IMQ application in mice was dependent on unintended oral IMQ ingestion and it is possible that some of the systemic effects observed in our study were elicited by systemic IMQ uptake rather than skin-driven inflammation
10.
The mechanisms responsible for differential effects of digoxin on IMQ-induced psoriasis-like skin inflammation on the ear and back in mice clearly require more study, but our results underline the complexity of the IMQ model and support caution in extrapolation of results of targeted interventions in this model to human psoriasis.
Figures and Tables
Fig. 1
Differential effects of digoxin on imiquimod (IMQ)-induced psoriasis-like skin inflammation on the ear and back. IMQ-induced skin thickening was assessed in the ear (A, B) and in the back skin (C, D). Thicknesses of the ear (A) and the back skin fold (C; left panel) were measured daily with a micrometer, from day 1 or 3 (A and C, respectively) until day 6. The back skin was visually scored for erythema and scaling each on a scale from 0~4 and the sum score (0–8) is presented (C; right panel). Values are presented as mean±standard deviation. Mean numbers of epidermal cell layers in the ear (B) and back skin (D) were assessed in H&E stained sections. Representative photos are also displayed (scale bar: 50 µm). Statistics were performed using two-way ANOVA with Tukey's correction for multiple comparisons. Due to technical difficulties, data from one mouse are missing from the vehicle cream (Veh)-control group (B) and from the Veh-digoxin group (D), respectively.
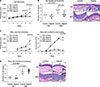
Table 1
Effects of digoxin on plasma concentrations of IL-17A and SAA and on spleen weight and BW

|
Total (n) |
|
Control |
Digoxin |
|
IL-17A (pg/ml) |
32 |
Veh |
5.36±2.06 |
6.05±2.28 |
|
IMQ |
19.05±5.22**
|
31.60±13.29****,†
|
|
SAA (μg/ml) |
28 |
Veh |
38.8±44.5 |
13.6±5.6 |
|
IMQ |
171.2±111.1*
|
221.6±98.8***
|
|
Spleen weight (mg/g BW) |
30 |
Veh |
3.21±0.74 |
3.14±0.60 |
|
IMQ |
8.06±1.00****
|
7.71±0.62****
|
|
BW (% of weight on day 1) |
31 |
Veh |
100.13±2.00 |
101.73±1.95 |
|
IMQ |
102.34±3.31 |
101.90±4.56 |
ACKNOWLEDGMENT
This work was supported by an unrestricted grant from the LEO Foundation, Industriparken 55, DK-2750 Ballerup, Denmark. The funder had no role in study design, data collection, data analysis and interpretation, manuscript preparation and/or publication decisions.
We thank Birgitte Sander Nielsen for excellent handling of mice, and Annette Blak Rasmussen and Kristine Møller for histological processing. We acknowledge the Core Facility for Integrated Microscopy, Faculty of Health and Medical Sciences, University of Copenhagen.
References
1. Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, et al. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol. 2013; 133:17–26.

2. van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009; 182:5836–5845.

3. Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006; 126:1121–1133.

4. Banerjee D, Zhao L, Wu L, Palanichamy A, Ergun A, Peng L, et al. Small molecule mediated inhibition of RORγ-dependent gene expression and autoimmune disease pathology in vivo. Immunology. 2016; 147:399–413.

5. Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, et al. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J Clin Invest. 2012; 122:2252–2256.

6. Skepner J, Ramesh R, Trocha M, Schmidt D, Baloglu E, Lobera M, et al. Pharmacologic inhibition of RORγt regulates Th17 signature gene expression and suppresses cutaneous inflammation in vivo. J Immunol. 2014; 192:2564–2575.

7. Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature. 2011; 472:486–490.

8. Shi H, Mao X, Zhong Y, Liu Y, Zhao X, Yu K, et al. Digoxin reduces atherosclerosis in apolipoprotein E-deficient mice. Br J Pharmacol. 2016; 173:1517–1528.

9. Schaper K, Dickhaut J, Japtok L, Kietzmann M, Mischke R, Kleuser B, et al. Sphingosine-1-phosphate exhibits anti-proliferative and anti-inflammatory effects in mouse models of psoriasis. J Dermatol Sci. 2013; 71:29–36.

10. Grine L, Steeland S, Van Ryckeghem S, Ballegeer M, Lienenklaus S, Weiss S, et al. Topical imiquimod yields systemic effects due to unintended oral uptake. Sci Rep. 2016; 6:20134.

SUPPLEMENTARY MATERIALS
Supplementary File 1
Detailed materials and methods






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download