This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
Rate of continence after artificial urinary sphincter (AUS) placement appears to decline with time. After appropriate workup to exclude inadvertent device deactivation, development of urge or overflow incontinence, and fluid loss, many assume recurrent stress urinary incontinence (rSUI) to be secondary to nonmechanical failure, asserting urethral atrophy as the etiology. We aimed to characterize the extent of circumferential urethral recovery following capsulotomy and that of pressure regulating balloon (PRB) material fatigue in men undergoing AUS revision for rSUI.
Materials and Methods
Retrospective review of a single surgeon database was performed. Cases of AUS removal/replacement for rSUI involving ventral subcuff capsulotomy and intraoperative PRB pressure profile assessments were identified.
Results
The described operative approach involving capsulotomy was applied in 7 patients from November 2015 to September 2017. Mean patient age was 75 years. Mean time between AUS placement and revision was 103 months. Urethral circumference increased in all patients after capsulotomy (mean increase 1.1 cm; range 0.5–2.5 cm). Cuff size increased, remained the same, and decreased in 2, 3, and 2 patients, respectively. Six of 7 patients underwent PRB interrogation. Four of these 6 PRBs (66.7%) demonstrated pressures in a category below the reported range of the original manufacturer rating.
Conclusions
Despite visual appearance to suggest urethral atrophy, subcuff capsulotomy results in increased urethral circumference in all patients. Furthermore, intraoperative PRB profiling demonstrates material fatigue. Future multicenter efforts are warranted to determine if capsulotomy, with or without PRB replacement, may simplify surgical management of rSUI with reductions in cost and/or morbidity.
Go to :

Keywords: Atrophy, Urinary incontinence, Urinary sphincter, artificial
INTRODUCTION
Male stress urinary incontinence (SUI) negatively affects quality of life. The AMS 800™ (Boston Scientific [formerly American Medical Systems], Minnetonka, MN, USA) artificial urinary sphincter (AUS) has been the gold standard of management since its release in the 1983 [
1]. Reported long-term outcomes vary, partly due to alternative definitions of continence. However, dryness is reported in 90% and 82% following primary and subsequent AUS placement, respectively [
2]. Furthermore, patient satisfaction rates are excellent, with overall satisfaction >90% [
3].
Rates of satisfaction and continence appear to decline with time, which is important for both patient counseling and efforts towards surgical quality improvement [
4]. Reported reoperation rates after AUS range from 14.8% to 44.8% [
5]. Indications for reoperation include infection with or without erosion, device malfunction or malpositioning, and/or recurrent SUI (rSUI). After appropriate workup to exclude inadvertent device deactivation, development of urge or overflow incontinence, and fluid loss from the system, many assume rSUI to be secondary to nonmechanical failure, asserting urethral atrophy as the etiology [
6].
Nonmechanical rSUI has prompted multiple innovations in surgical management. Tandem cuff placement was initially found to have lower rates of rSUI than other forms of AUS revision, including cuff downsizing and repositioning [
78]. Yet, longer follow-up for tandem cuffs demonstrated a higher complication rate, with one concern being erosion at the distal cuff [
9]. Others have proposed circumferential urethral bulking by including bulbospongiosus muscle or a commercially available biologic ‘wrap’ between the AUS cuff and urethra [
1011]. These options involve off-label technique and products with often unknown long-term outcomes. Furthermore, transcorporal AUS placement has been shown to be effective, but may compromise chances for subsequent penile prosthesis placement, if desired [
1213].
More recently, our practice has changed to include ventral subcuff capsulotomy at time of AUS revision for cases of rSUI in hopes of restoring urethral circumference so as to avoid cuff downsizing, need for transcorporal or tandem cuff placement, or need for further urethral dissection and, the purpose of this study, was to assess if this was feasible. In addition to releasing the fibrotic ‘waist’ constricting the urethra, we have performed intraoperative assessments to determine the pressure profile of the pressure regulating balloon (PRB). The rationale for this maneuver was to determine if material fatigue, over time, could potentially represent an additional factor in failure to occlude the urethra between voiding episodes.
Go to :

MATERIALS AND METHODS
We retrospectively reviewed a prospectively collected, Institutional Review Board (IRB)-approved database of urologic prosthetic surgery performed by a single reconstructive urologist (Wake Forest Baptist Medical Center; approval number: IRB00042919). The described operative approach involving capsulotomy was applied in all 7 patients who underwent AUS revision surgery from November 2015 to September 2017 at Wake Forest Baptist Medical Center (Winston-Salem, NC, USA). All patients with rSUI were evaluated for bladder dysfunction, device malfunction, and the possibility of urethral erosion via office-based flexible cystourethroscopy.
Cases managed with the technique presented herein underwent initial circumferential urethral measurement following cuff uncoupling. Next, the capsule was incised ventrally, along the vertical access, with dissection to free the urethra from the overlying fibrous tissue. Time was allowed for intraoperative observation of urethral expansion before measurement. Complete capsulectomy was not performed to avoid any undue disruption of urethral integrity. Repeat circumferential measurement was performed to allow appropriate cuff size selection. This was done at the site of the prior cuff using a vessel loop and/or commercially-available measuring tape.
Explanted PRBs were interrogated after measuring the extracted saline volume and verifying absence of fluid loss and/or inadvertent perforation. After refilling intact PRBs with 24 mL of sterile saline, the device was connected to a manometer used for arterial monitoring with appropriate reference, and the reading was recorded. Values were converted from mm Hg to cm H2O and compared to manufacturer ratings associated with the original product. All cases in this series received an entirely new system placed in standard fashion featuring PRBs rated for pressures between 61–70 cm H2O.
Data related to patient demographics, prior therapies, and outcomes after revision were collected and analyzed.
Go to :

RESULTS
The described operative approach involving capsulotomy was applied in all 7 patients undergoing AUS revision from November 2015 to September 2017 (
Fig. 1). Mean patient age was 75. Mean time between original AUS placement and AUS revision surgery was 103 (range 24–205) months. Six patients underwent prior radical prostatectomy and the remaining patient underwent prior transurethral resection of prostate followed by brachytherapy. Urethral circumference increased in all patients after capsulotomy (mean increase, 1.1 cm; range, 0.5–2.5 cm). Relative to the prior product, replacement cuff size was either unchanged or increased in 71.4% (5/7) patients. Of the 2 patients with a subsequent decrease in cuff size, 1 was a prior transcorporal cuff and both cuffs decreased by only 0.5 cm.
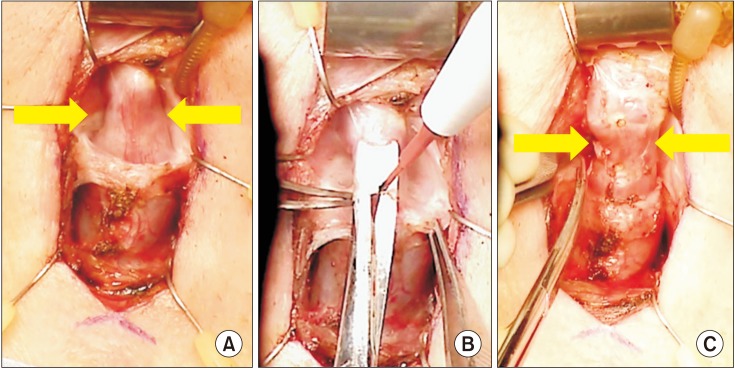 | Fig. 1(A) Urethra at site of initial cuff placement prior to capsulotomy with 3 cm urethral circumference. (B) Ventral capsulotomy performed via combination of sharp dissection and electrocautery. (C) Urethra at site of initial cuff placement after capsulotomy measuring 4 cm in urethral circumference, demonstrating restoration of urethral circumference.
|
All patients had PRBs rated for 61 to 70 cm H2O at both initial and subsequent placement. Six of the 7 patients underwent PRB interrogation. The 66.7% (4/6) of PRBs demonstrated pressures below the range listed for the original manufacturer rating. Of note, all of these cases were referred after having undergone primary AUS placement by outside providers.
An additional patient underwent removal and replacement of PRB without cuff replacement 6 months after initial AUS surgery for PRB herniation. The prior PRB was rated for a pressure of 61 to 70 cm H
2O, but interrogation revealed a pressure of only 58.5 cm H
2O. Cuff size selection relative to that of the initial operation, as well as the results of PRB interrogations are shown in
Table 1.
Table 1
Urethral circumference, AUS cuff size, and PRB interrogation pressures
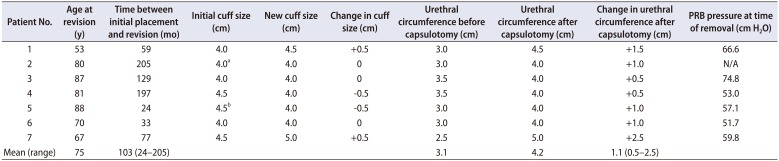
|
Patient No. |
Age at revision (y) |
Time between initial placement and revision (mo) |
Initial cuff size (cm) |
New cuff size (cm) |
Change in cuff size (cm) |
Urethral circumference before capsulotomy (cm) |
Urethral circumference after capsulotomy (cm) |
Change in urethral circumference after capsulotomy (cm) |
PRB pressure at time of removal (cm H2O) |
|
1 |
53 |
59 |
4.0 |
4.5 |
+0.5 |
3.0 |
4.5 |
+1.5 |
66.6 |
|
2 |
80 |
205 |
4.0a
|
4.0 |
0 |
3.0 |
4.0 |
+1.0 |
N/A |
|
3 |
87 |
129 |
4.0 |
4.0 |
0 |
3.5 |
4.0 |
+0.5 |
74.8 |
|
4 |
81 |
197 |
4.5 |
4.0 |
−0.5 |
3.5 |
4.0 |
+0.5 |
53.0 |
|
5 |
88 |
24 |
4.5b
|
4.0 |
−0.5 |
3.0 |
4.0 |
+1.0 |
57.1 |
|
6 |
70 |
33 |
4.0 |
4.0 |
0 |
3.0 |
4.0 |
+1.0 |
51.7 |
|
7 |
67 |
77 |
4.5 |
5.0 |
+0.5 |
2.5 |
5.0 |
+2.5 |
59.8 |
|
Mean (range) |
75 |
103 (24–205) |
|
|
|
3.1 |
4.2 |
1.1 (0.5–2.5) |
|

Mean time from revision surgery to last clinical follow-up was 11.8 months (range, 2.0–25.2 months). Dry rate among AUS revision patients, defined as 0 to 1 pad per day, was 71.4% (5/7). No complications were encountered in any patient.
Go to :

DISCUSSION
The concept of the subcuff capsule has been mentioned by others, but our simplistic approach of ventral capsulotomy rather than full capsulectomy has not been previously reported [
14]. Additionally, our data is the first to demonstrate restoration of urethral circumference, lending support to the notion that prosthetic urologists may safely avoid further urethral dissection and its associated disruptions in collateral vascularity at time of revision surgery. Also, avoiding more proximal mobilization reduces the risk of inadvertent dorsal urethrotomy, as the urethra becomes more eccentrically located within the corpus spongiosum. The results reported here suggest that the need to downsize cuffs may be overstated within the literature. Use of the three pillowed 3.5 cm AUS cuff has been shown to have a high revision rate and was abandoned in our practice [
15].
We do acknowledge the concept that the capsule could recur, but this is true regardless of how one approaches the operation. Thus, placing the new cuff in the same location as the previous cuff is an approach that potentially ‘burns fewer bridges’. Future scientific investigation into coatings or other agents capable of reducing the degree and/or density of physiologic encapsulation likewise seems warranted. Furthermore, the PRB was noted to have a reduced pressure profile in most cases. However, it is unknown if the pressure applied may have been higher in vivo secondary to PRB encapsulation.
This study is not, however, without limitations. This was a retrospective review of a small cohort of patients and may or may not be representative of the general population. Longer-term follow-up is necessary to determine durability of this approach and a multicenter study may afford stronger data. This series is of insufficient volume to assert efficacy for continence, but offers proof of concept that the urethra is constricted by external compression, rather than a matured atrophic process within the spongiosum. Future surgical management in the setting of rSUI after AUS may attempt to initially preserve the tab securing the cuff to determine if simple capsulotomy (with or without PRB exchange) can rescue effective urethral closure with the existing device. This could simplify the nature of revision surgery, with potential reductions in operative time, cost, and time to activation. The traditional, and admittedly dogmatic, six-week waiting period from initial placement to activation seemingly serves to avoid manipulation while tissue recovery takes place around the device, securing the pump and cuff in position. Following capsulotomy, if the initial device could simply be recoupled, the traditional waiting period may not be necessary.
Go to :

CONCLUSIONS
Our experience suggests that, although intraoperative visualization at AUS revision may suggest an ‘atrophied’ urethra, this appears to be an artifact of subcuff encapsulation. Capsulotomy allowed urethral recovery for all cases in this series. Feasibility of using a similarly sized cuff relative to that of the initial operation with adequate occlusion following capsulotomy suggests revision surgery may be simplified in the future. Revision cases are likely best served by experts well-versed in urethral and prosthetic surgery. Future multicenter clinical efforts are warranted to substantiate our findings.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download