This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
Intravesical electrical stimulation treatment (IVES) has been successfully used to treat neurogenic bladder. We report the results of an observational study regarding the use of IVES for women with overactive bladder syndrome (OAB) and/or urgency urinary incontinence (UUI).
Materials and Methods
IVES was performed in women with OAB (defined by frequency ≥8/day, nocturia ≥2/night, or ≥3 episodes of UUI on 3-day voiding diary) who failed prior medical therapy. Subjects underwent 4 weeks of treatment with an 8-Fr Detruset™ IVES catheter. Primary outcome was Patient Global Impression of Improvement (PGI-I) at 3 months. Secondary outcomes included Visual Analog Scale (VAS), Short Form OAB Questionnaire (OAB-q SF), Pelvic Floor Distress Inventory (PFDI), Pelvic Floor Impact Questionnaire (PFIQ), reduction in frequency and UUI on voiding diary, and adverse effects. Analysis was done with paired t-tests and Wilcoxon signed rank tests.
Results
Seventeen subjects completed the study. At 4 weeks post-treatment, 15 improved on PGI-I (11 subjects: ‘a little better’, 2: ‘much better’, 2: ‘very much better’). There were significant improvements in symptom bother and health-related quality of life as measured by OAB-q SF and pelvic organ prolapse and urinary distress as measured by PFDI. Frequency decreased from 10.3±4.3 at baseline to 8.9±2.3 (p=0.04) at 3 months. No pain was reported during treatment. There was one urinary tract infection during the study period. No other adverse events were reported.
Conclusions
IVES appears to be a safe and effective novel treatment for OAB. Larger comparative studies are needed to investigate its potential for long-term treatment.
Go to :

Keywords: Electrical stimulation, Urinary bladder, overactive, Urinary incontinence, urge
INTRODUCTION
Overactive bladder (OAB) is a very common clinical condition, with an overall prevalence estimated at 11.8% in the general population [
1]. The American Urological Association Guidelines recommend behavioral therapy as first-line treatment to be followed by oral pharmacologic treatment such as anticholinergic or β
3-agonist therapy as a second-line treatment [
2]. For a subset of patients these medications are either not efficacious or poorly tolerated dueto side effects. Percutaneous tibial nerve stimulation (PTNS), intravesical onabotulinum toxin A injection, and sacral neuromodulation are considered the standard treatment options for these patients.
Both PTNS and sacral neuromodulation utilize electrical stimulation to provide OAB relief and have been demonstrated in randomized controlled trials to be efficacious in the treatment of refractory OAB [
345]. PTNS is thought to be less invasive but also seems to be less potent than sacral neuromodulation. Intravesical electrical stimulation (IVES) was developed to stimulate the bladder directly via transurethral catheter and it has been questioned if this can be similar to two established treatment modalities for refractory OAB in adults. Allthese modalities utilize electrical stimulation to provideOAB relief; however, they vary according to the anatomicalstructure in which the electrical stimulation is applied.
IVES has long been studied for the treatment of lower urinary tract dysfunction in certain populations, specifically pediatric populations with spinal dysraphisms or spinal cord injury and resultant neurogenic bladder [
678]. To date, there has been limited study examining the use of IVES for the treatment of OAB in the neurologically intact adult human.
Go to :

MATERIALS AND METHODS
After receiving approval from the Loma Linda University Medical Center Institutional Review Board (approval number: 5130321), from March 2014 to March 2015, patients with OAB were recruited to take part in our study. OAB was defined as frequency ≥8/day, nocturia ≥2/night or ≥3 episodes of urgency urinary incontinence (‘OAB-wet’) during a 3-day voiding diary. All subjects participating in the study failed prior medical treatment with more than one medication, including anticholinergics and β3-agonists. The reason for discontinuation was either inadequate efficacy or significant side effects. Subjects with neurogenic bladder, nocturnal polyuria, mixed urinary incontinence with a predominantly stress incontinence component, Baden-Walker stage 2 or greater pelvic organ prolapse, unresolved urinary tract infection (UTI), vesicoureteral reflux, bladder calculi or malignancy, body mass index (BMI)>35 kg/m2, pacemaker or defibrillator in situ, or recent OAB treatment occurring within one month of recruitment were excluded. Women who were pregnant or taking anticoagulant, immunosuppressant, or peripheral neuropathy medications were also excluded.
A disposable 8 Fr Detruset™ catheter connected to a Detrusan 500™ electrical generator (EMED Technologies, El Dorado Hills, CA, USA) was used to perform IVES treatments twice/week for 4 weeks. Program number 4 on the Detrusan 500™, which delivers a frequency of 5 Hz and pulse width of 200 µs, was used for the first 5 minutes of treatment. The subsequent 15 minutes of treatment were performed with program number 12, which delivers a frequency alternating between 50 Hz and 5 Hz with a pulse width of 350 µs. The programs were based on the company's recommended settings and anecdotal experience of a co-author from the past. The voltage was adjusted at the beginning of the procedure to the maximum voltage at which the patient was comfortable. All such voltage adjustments were made within the first minute after the electrodes were energized.
The primary outcome was the patient global impression of improvement scale (PGI-I). Secondary outcomes included the symptom score on visual analogue scale (VAS), Overactive Bladder Questionnaire Short Form (OAB-q SF), Pelvic Floor Distress Inventory (PFDI), Pelvic Floor Impact Questionnaire (PFIQ), reduction in urinary frequency and urgency incontinence episodes during a 3-day voiding diary, and adverse effects including UTI based on urinalysis.
Patients were in dorsal lithotomy position and the Detruset™ catheter was placed into the bladder. They were instructed to come with relatively full bladder by avoiding urination winthin at least 2 hours before treatment. The generator was activated and a 20 minute treatment session was administered. This was repeated twice/week for 4 weeks. The first five patients in the study also underwent cystoscopy prior to and after the completion of their treatment in order to check for signs of mucosal injury.
Subjects completed the above-mentioned questionnaires prior to IVES treatment and also at follow-up visits at 2, 3, 4, and 12 weeks after the initial treatment. They were seen again in clinic at 3-month intervals thereafter and completed the questionnaires at these visits as well. Statistical analysis was performed using Statistics Analysis System ver. 9.4 (SAS Institute Inc., Cary, NC, USA) using paired t-tests and non-parametric Wilcoxon signed rank tests with p<0.05 considered statistically significant.
Go to :

RESULTS
Seventeen subjects were recruited after they were screened for inclusion and exclusion criteria. The mean voltage settings used in program number4 was 25.07±5.00 V. The mean voltage settings used in program number12 was 23.02±6.00 V. All 17 subjects were included for analysis. The mean age was 60.8 years (range, 29–74 years). Mean BMI was 31.1 kg/m2 (range, 20.4–53.2 kg/m2). Subjects reported a baseline bother score of 8.1, OAB-q SF (health-related quality of life, HRQL) of 49.1, and OAB-q SF (symptom bother) of 24.6. Subjects reported a mean baseline urinary frequency of 10.3 episodes/day, 3.0 episodes of urgency urinary incontinence, and use of 2.9 pads/day during a 3-day voiding diary. Out of 17 subjects, six subjects met the definition of ‘OAB-wet’.
All subjects completed the full 4-week treatment course of IVES. No pain was reported during the treatment session. Apart from a single episode of UTI, no adverse events were reported. No abnormalities were noted on cystoscopy.
At the conclusion of treatment (4 weeks after the first treatment), 15/17 subjects reported improvement on PGI-I. Eleven subjects reported that symptoms were ‘a little better’, 2 reported that they were ‘much better’, and 2 reported that they were ‘very much better’. One patient reported that there was ‘no change’ and one reported that symptoms were ‘a little worse’ (
Fig. 1). The mean number of pads used per day decreased from 2.9 to 2.0. This approached but did not reach statistical significance (p=0.08). At 8 weeks after the conclusion of treatment (12 weeks after the first treatment), 14/17 subjects (82.4%) reported improvement on PGI-I. Ten subjects reported that symptoms were ‘a little better’, 3 reported they were ‘much better’, and 1 reported that they were ‘very much better’. One patient reported that there was ‘no change’ in symptoms, one reported that they were ‘a little worse’, and one reported that they were ‘very much worse’ (
Fig. 1).
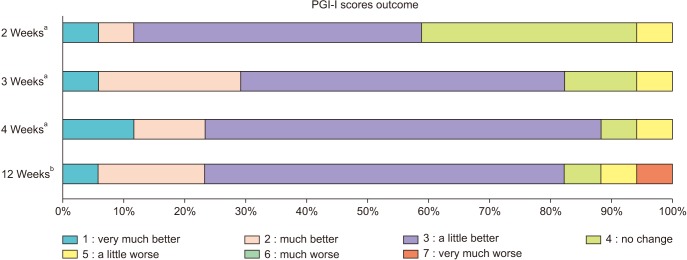 | Fig. 1Patient global impression of improvement scales (PGI-I) at 2, 3, 4, and 12 weeks after first treatment. a:Time from the first treatment. b:Equals to 8 weeks from the end of 4 weeks treatment.
|
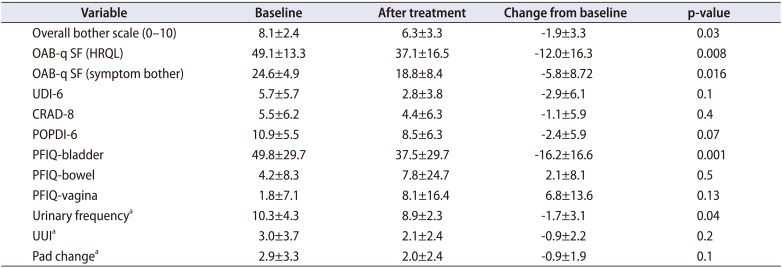
Patients also reported statistically significant improvements in overall bother scale, OAB-q SF (HRQL), OAB-q SF (symptom bother), PFIQ-bladder, and urinary frequency at 12 weeks after IVES treatment (
Table 1). OAB-q-SF mean score fell from baseline of 49 to a low of 33 and OAB-q SF fell from 24 at baseline to a low of 16 by week 4 (
Fig. 2). The pelvic organ prolapse distress inventory-6 (POPDI-6), colorectal anal distress inventory-8, and urogenital distress inventory-6 (UDI-6) scores all trended toward improvement with maximal benefit seen at weeks 3 and 4, however this was not statistically significant (
Fig. 3). Mean PFIQ-bladder scores improved from a baseline of 50 to a low of 37 at week 3, and this improvement was sustained out to week 12, however there was a slight worsening in PFIQ-bowel and vagina scores after treatment (
Fig. 4). Overall, the mean follow-up was 4.9 months (range, 3–12 months).
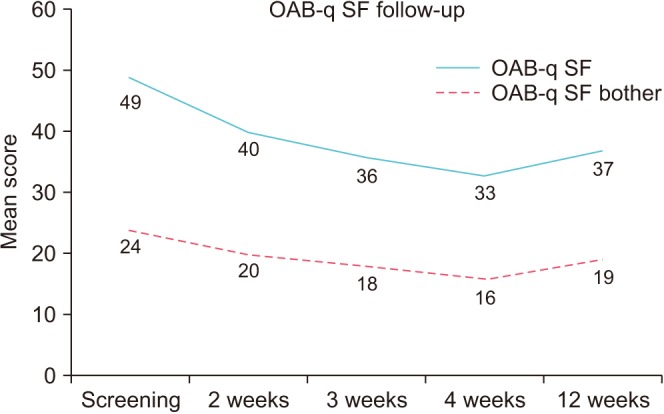 | Fig. 2Overactive bladder symptom bother and health related quality of life outcomes at baseline and at 2, 3, 4, and 12 weeks after treatment. OAB-q SF, Overactive Bladder Questionnaire Short Form.
|
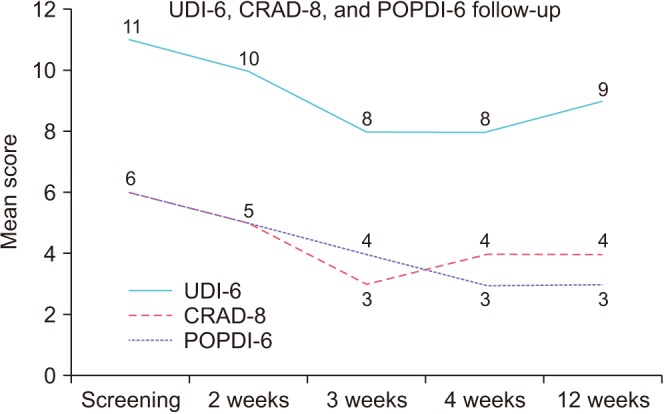 | Fig. 3Pelvic organ prolapse distress inventory-6 (POPDI-6), urogenital distress inventory-6 (UDI-6), and colorectal anal distress inventory-8 (CRAD-8) outcomes at baseline and at 2, 3, 4, and 12 weeks after treatment.
|
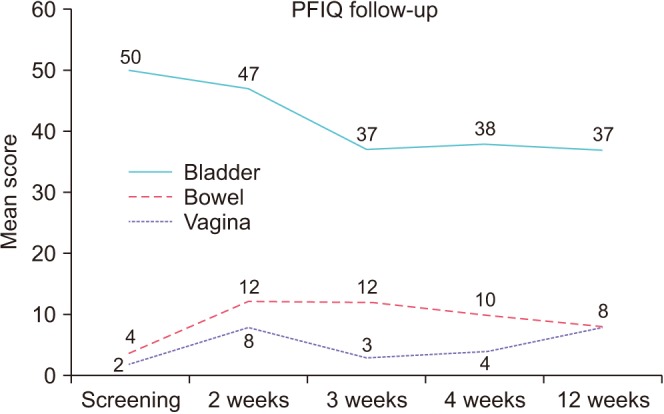 | Fig. 4Pelvic Floor Impact Questionnaire (PFIQ)-7 at baseline and at 2, 3, 4, and 12 weeks after treatment.
|
Table 1
Outcomes at 12 weeks after intravesical electrical stimulation treatment
|
Variable |
Baseline |
After treatment |
Change from baseline |
p-value |
|
Overall bother scale (0–10) |
8.1±2.4 |
6.3±3.3 |
−1.9±3.3 |
0.03 |
|
OAB-q SF (HRQL) |
49.1±13.3 |
37.1±16.5 |
−12.0±16.3 |
0.008 |
|
OAB-q SF (symptom bother) |
24.6±4.9 |
18.8±8.4 |
−5.8±8.72 |
0.016 |
|
UDI-6 |
5.7±5.7 |
2.8±3.8 |
−2.9±6.1 |
0.1 |
|
CRAD-8 |
5.5±6.2 |
4.4±6.3 |
−1.1±5.9 |
0.4 |
|
POPDI-6 |
10.9±5.5 |
8.5±6.3 |
−2.4±5.9 |
0.07 |
|
PFIQ-bladder |
49.8±29.7 |
37.5±29.7 |
−16.2±16.6 |
0.001 |
|
PFIQ-bowel |
4.2±8.3 |
7.8±24.7 |
2.1±8.1 |
0.5 |
|
PFIQ-vagina |
1.8±7.1 |
8.1±16.4 |
6.8±13.6 |
0.13 |
|
Urinary frequencya
|
10.3±4.3 |
8.9±2.3 |
−1.7±3.1 |
0.04 |
|
UUIa
|
3.0±3.7 |
2.1±2.4 |
−0.9±2.2 |
0.2 |
|
Pad changea
|
2.9±3.3 |
2.0±2.4 |
−0.9±1.9 |
0.1 |

Go to :

DISCUSSION
The pathophysiology underlying adult OAB is complex and multifactorial. Indeed, there are likely to be multiple different derangements in bladder neural transmission that can cause the OAB symptom complex. This would explain why patients with seemingly similar OAB symptoms may respond to different forms of treatment. Further study is needed to further elucidate these mechanisms and the way that IVES as well as other existing OAB treatments can act on them. One particular area of interest would be with regards to suprapontine and spinal cord input into the micturition reflex arc and whether this input is affected by IVES.
Much of the existing literature regarding IVES involves its use in pediatric patients with neurogenic bladder. Although this patient population is clearly very different from the adult OAB population, there may be commonalities in terms of underlying pathophysiology. Sensitization of C-fiber sensory afferents resulting in detrusor overactivity has been proposed as a possible etiology in both patient populations [
910]. A recent study of middle-aged women demonstrated an age-related decrease in the current perception threshold and latency as well as an increase in the amplitude of sensory evoked potentials in the bladder dome, trigone, and proximal urethra. The authors interpreted this as evidence of an age-related increase in bladder afferent excitability which may contribute to the age-related increase in OAB [
11].
IVES in a rat spinal cord injury model has been shown to decrease both the number of nonvoiding detrusor contractions and maximal detrusor pressure [
12]. A recent retrospective study of spina bifida children found that IVES can increase urodynamic bladder capacity. Detrusor overactivity was improved in 41.7% of patients and bladder capacity was improved in 45.6% [
6]. A previous multi-institutional study of 568 children with neurogenic bladder showed that 53% of patients had an increase in bladder capacity of 20% or greater over 1.9 years of follow-up [
13] while a separate retrospective study of 405 children with neurogenic bladder demonstrated that 76.9% of patients had a 20% or greater increase in bladder capacity over 6.6 years [
14].
The data from the voiding diary showed statistically significant reduction in urinary frequency (p=0.04) but the clinical significance of 1.7 less frequency per a day can be questioned. PFIQ is a quality of life indicator in three different domains of bladder, bowel, and vagina. Apparently bladder score improved significantly after IVES treatment but other two domain scores increased slightly (
Fig. 4). However, it was not statistically significant. It is not clear if it has any clinical meanings. As we targeted OAB population, the subjects had much higher scores in bladder domain compared to bowel or vagina as a baseline.
This study shows that IVES could potentially find a role in the treatment of patients with refractory OAB. IVES is advantageous in that it is less invasive and does not require anesthesia, as is the case for sacral neuromodulation. In addition, the rate of side effects such as UTI or urinary retention seems to be much lower than for intravesical onabotulinum toxin injection. As a less invasive treatment with a relatively low rate of side effects, IVES seems to share some similarities with PTNS treatment and may occupy a similar space within the treatment algorithm for refractory OAB. Anecdotally, among those who had both IVES and PTNS in our urogynecology clinic, some patients experienced superior results with IVES compared to PTNS whereas the opposite was true for other patients. If the efficacy of IVES is established with future studies, comparative studies between IVES and PTNS will need to be carried out.
One limitation is the lack of a control group in our study, given that prior studies of OAB treatment regimens have demonstrated a significant placebo effect. A randomized controlled trial with a control arm in which patients are given sham placebo treatment compared to a treatment arm in which patients are given IVES would be ideal. Another limitation of our study is the follow-up period. While a mean follow-up duration of 4.9 months is not insignificant, studies with longer follow-up are certainly indicated to demonstrate a lasting effect. It has been shown that a 6 week pause of maintenance PTNS treatment reduced the efficacy significantly [
15]. Similarly, intravesical onabotulinum toxin injection for refractory OAB can require repeat treatment at 6 to 12 months intervals. Finally, our study is limited by its small sample size. Due to small sample size, we were not able to demonstrate any patient characteristics which affects the treatment outcome with statistical significance. And eventually a large, randomized controlled trial will be necessary to confirm the studies result, and elucidate the optimal treatment schedule and necessity of maintenance treatments.
Despite these limitations, this study does provide a promising result in that improved outcome was demonstrated using standardized validated criteria. Apart from a single episode of UTI, there were no adverse effects noted during the study period and all subjects completed the treatment and cystoscopy showed no abnormalities or changes post-procedure. Our study population did not have severe OAB, in terms of number of urinary frequency and urgency incontinence, and the efficacy of treatment may be more pronounced in patients with severe OAB. In addition, the primary outcome measure showed a significant reduction in OAB symptoms even two months after the completion of treatment. This sustained efficacy is encouraging. To our knowledge, this study represents the first report in the scientific literature regarding the use of IVES in the neurologically intact adult OAB population. Future studies will need to elucidate the appropriate treatment schedule and generator settings and also provide higher-level evidence in the form of randomized controlled trials comparing IVES to a sham treatment and to existing OAB treatments.
Go to :

CONCLUSIONS
Administration of IVES was associated with a statisticallysignificant improvement in OAB symptoms asmeasured by validated questionnaires in a short-termobservational study. Self-reported urinary frequency andpad usage also improved. This treatment was well-tolerated.This opens up the need for further studies to investigate thepotential use of this treatment modality for OAB patients.
Go to :









 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download