This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
To evaluate the expression of estrogen receptor (ER)-beta and Ki67 in prostate cancer and study their relationship.
Materials and Methods
We analyzed 101 cases of prostate adenocarcinoma diagnosed from January 2011 to June 2015 in 100 patients. Immunohistochemical staining of ER-beta and Ki67 was analyzed according to Gleason score categorized into prognostic groups of 1 to 5. Double-immunofluorescent staining of ER-beta and Ki67 was performed in a total of 20 cases to study the co-expression and the relationship between these markers within the same tumor.
Results
A total of 53 of 101 cases (52.5%) were positive for ER-beta expression. There was a positive correlation whereby a high percentage of ER-beta expression was seen in the higher prognostic groups (groups 4 and 5; p=0.007). High Ki67 expression was observed in the higher prognostic group, whereas low Ki67 or negative expression was found in the lower prognostic group (p<0.001). The majority of cases evaluated with double-immunofluorescent staining (14/20) showed co-expression of ER-beta and Ki67 at the individual cell level.
Conclusions
ER-beta and Ki67 are independent tumor markers in high prognostic groups. Hence, co-expression of ER-beta and Ki67 indicates a more aggressive tumor with a poorer prognosis.
Go to :

Keywords: Estrogen receptor beta, Ki-67 antigen, Neoplasm grading, Prostate neoplasms
INTRODUCTION
Prostate cancer (PCa) is the second most common malignancy and the fifth leading cause of cancer death in men with a total of 1.09 million new PCa cases and 307,500 disease-related deaths in 2012 [
1]. In Malaysia, PCa is the fifth most common cancer among Malaysian men [
2]. The incidence of PCa increases with age, and the disease is most often diagnosed after the age of 65 years. Hence, 60% of cases are detected at a late stage (stages 3 and 4), with only 16% at stage 1 and 24% at stage 2 [
2].
Estrogen receptor (ER)-beta is the predominant ER in normal prostate and is preferentially expressed in epithelial cells [
3]. The role of ER-beta in the pathogenesis or prognosis of PCa is unclear, however. It has been suggested that it controls proliferation and prevents hyperplasia in rodent prostate because ER-beta knockout mice show prostatic hyperplasia with aging and high epithelial proliferation with a low apoptosis index [
4]. In the absence of ER-beta, the epithelium does not fully differentiate and a large fraction of epithelial cells retain their capacity to proliferate [
4]. Few studies have examined the variety of pattern of ER expression in prostate tumor. One previous study revealed that ER-beta expression is reduced in higher-grade prostatic adenocarcinoma compared with low- and intermediate-grade prostatic adenocarcinoma [
5]. It has also been shown that ER-beta is highly expressed in normal human prostate and that there is a progressive loss of expression in prostatic hyperplasia and to a greater extent in invasive cancer [
6]. Evidence also exists of increased ER staining intensity in malignant prostate [
7]. It has been postulated that ER-beta switches its role during cancer progression. In the early phases of PCa progression, ER-beta presents as a tumor-suppressing agent and then becomes a tumor promoter as cancer progresses. It may also have a role in the process of prostatic hyperplasia and malignancy [
89]. The expression of this biomarker may thus be useful as a predictive marker in tumor staging and prognosis and may also shed new light on targeted treatment in prostate adenocarcinoma.
Ki67 is a protein found in proliferating cells. Ki67 recognizes a proliferation-specific nuclear antigen. Normal prostate cells proliferate slowly with low Ki67 expression. In prostate tumors, high Ki67 expression is associated with a higher Gleason score and more aggressive cancer [
101112]. Data from previous reports (on individual markers) suggest that ER-beta is absent or down-regulated in high-grade prostate adenocarcinoma and that Ki67 is highly expressed in highgrade prostate adenocarcinoma. However, no studies have looked at the co-expression or relationship between ER-beta and cell proliferation within the same tumor. In the present study, we investigated the expression of ER-beta and Ki67 and the relationship with the proliferation rate in PCa of various Gleason scores.
Go to :

MATERIALS AND METHODS
This study was conducted in Universiti Kebangsaan Malaysia Medical Center, Malaysia. Ethical approval was obtained from the local ethics committee (reference number FF-2014-146). A total of 101 paraffin-embedded blocks from cases of prostate adenocarcinoma of different Gleason grades from 100 patients diagnosed from January 2011 to June 2015 were retrieved from the department archive along with their corresponding H&E-stained slides. The PCa tissues were obtained from transrectal biopsy and transurethral resection of the prostate. Representative areas of prostate adenocarcinoma were marked on each H&Estained slide and the corresponding areas on paraffin blocks were cored for construction of tissue microarrays (TMAs) as well as for full tissue sections. A total of 46 cases were constructed for TMAs and 55 cases were used for full tissue sections. Representative 3-µm paraffin sections were made for immunohistochemical staining for ER-beta and Ki67.
Double-immunofluorescent staining was performed on a total of 20 cases, including 14 cases of prostate adenocarcinoma showing strong positivity for ER-beta with a high Ki67 proliferation rate, 4 cases showing weak positivity for ER-beta with a high Ki67 proliferation rate, and 2 cases showing weak positivity for ER-beta with a low Ki67 proliferation rate.
Mouse monoclonal antibody against Ki67 was used at a dilution of 1:100 with normal tonsil tissue as the positive control. Rabbit monoclonal antibody against ER-beta was used at a dilution of 1:300 with human ovarian serous cystadenocarcinoma as the positive control.
Immunohistochemistry staining was performed by using the protocol from EnVision™ FLEX+(mouse, high pH; Dako, Santa Clara, CA, USA). Primary antibodies were diluted to optimal concentration using Dako REAL™ Antibody Diluent (Dako). Tissue blocks were sectioned and an initial deparaffinization and pre-treatment step was performed in the Dako PTLink using the EnVision™ FLEX Target Retrieval Solution (Dako), high pH, followed by cooling at room temperature for 20 minutes and rinsing under running tap water for 3 minutes. The slides were subsequently incubated with EnVision™ FLEX peroxidase-blocking reagent for 5 minutes followed by the washing step. Slides were then incubated with primary antibody at room temperature followed by incubation with EnVision™ FLEX/HRP (Dako) for 20 minutes. Sections were then incubated with 1X DAB-containing substrate working solution for 10 minutes and then counterstained with hematoxylin.
Double-immunofluorescent staining was performed at optimal concentrations of primary and secondary antibodies. After deparaffinization and pre-treatment, the slides were incubated for 45 minutes at room temperature with the primary antibody mixture, washed with TBS for 10 minutes, and incubated in the dark moist chamber with a mixture of 2 isotype-specific secondary antibodies conjugated to Alexa Fluor 594® (red) and Alexa Fluor 488® (green) for 45 minutes. The slides were then washed with TBS for 30 minutes, followed by a counterstaining step using DAPI II (Abbott Molecular Inc., Des Plaines, IL, USA). Human ovarian cystadenocarcinoma tissue was used as a positive control.
Sections stained for ER-beta were scored qualitatively and those stained for Ki67 were scored quantitatively at ×40 magnification. ER-beta was interpreted as positive when the cells showed nuclear immunoreactivity in 10% or more of the tumor cells (
Fig. 1). Negative staining was interpreted if no staining was seen or when less than 10% of tumor cells showed positive expression regardless of staining intensity. Results for Ki67 were interpreted as negative if there was a lack of nuclear immunoreactivity (no staining at all), low in cases with positive nuclear staining of less than 10% (
Fig. 2) of the cells, and high in cases with positive nuclear staining of 10% or more of the cells (
Fig. 3).
 | Fig. 1Strong positive staining pattern for estrogen receptor beta (immunohistochemical staining, ×20).
|
 | Fig. 2Low Ki67 staining pattern. Strong positive staining pattern for estrogen receptor beta (immunohistochemical staining, ×10).
|
 | Fig. 3High Ki67 staining pattern. Strong positive staining pattern for estrogen receptor beta (immunohistochemical staining, ×20).
|
For double-immunofluorescent staining, ER-beta and Ki67 expression was detected in the nuclei of tumor cells. The nuclei that were positive for ER-beta were stained red and those positive for Ki67 were stained green. Nuclei with ER-beta and Ki67 co-expression appeared yellowish.
The slides were reviewed by two observers to determine the Gleason score and interpret the immunohistochemical and double-immunofluorescent staining. The cases were categorized and analyzed according to prognostic Gleason grade grouping of group 1 to group 5 (group 1, Gleason score of less than or equal to 6; group 2, Gleason score of 3+4; group 3, Gleason score of 4+3; group 4, Gleason score of 8; and group 5, Gleason scores of 9 and 10).
Statistical analysis was performed using IBM SPSS ver. 22.0 software (IBM Co., Armonk, NY, USA). Chi-square and Fisher's exact test were used for comparative analysis. The p-values of <0.05 were considered statistically significant.
Go to :

RESULTS
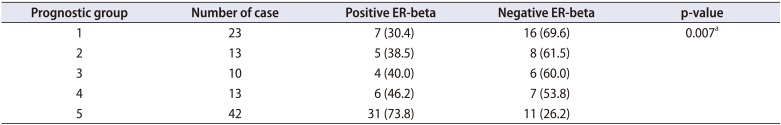
A total of 53 of 101 cases (52.5%) were positive for ER-beta expression at variable levels of intensity. ER-beta was observed in 7 of 23 cases (30.4%) from group 1, 5 of 13 cases (38.5%) from group 2, 4 of 10 cases (40.0%) from group 3, 6 of 13 cases (46.2%) from group 4, and 31 of 42 cases (73.8%) from group 5 (
Table 1).
Fig. 4 shows the number of positive cases according to prognostic group. Statistical analysis with the Pearson chi-square test showed a correlation or association between ER-beta expression and high prognostic group in which a high percentage of ER-beta positivity was found in the high prognostic group (p=0.007;
Table 1).
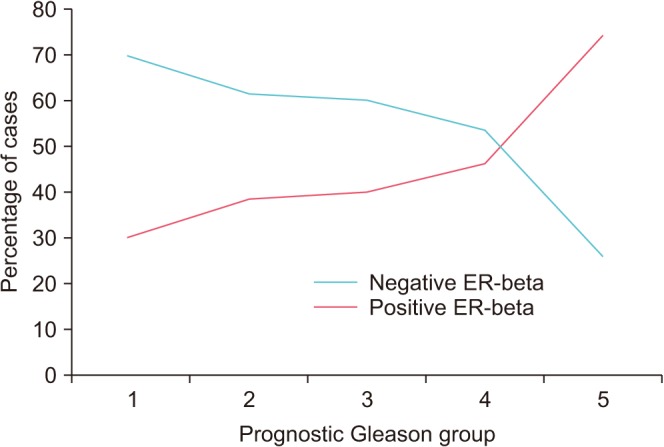 | Fig. 4Pattern of estrogen receptor (ER)-beta expression in relation to prognostic group.
|
Table 1
ER-beta expression in relation to prognostic Gleason group
|
Prognostic group |
Number of case |
Positive ER-beta |
Negative ER-beta |
p-value |
|
1 |
23 |
7 (30.4) |
16 (69.6) |
0.007a
|
|
2 |
13 |
5 (38.5) |
8 (61.5) |
|
|
3 |
10 |
4 (40.0) |
6 (60.0) |
|
|
4 |
13 |
6 (46.2) |
7 (53.8) |
|
|
5 |
42 |
31 (73.8) |
11 (26.2) |
|

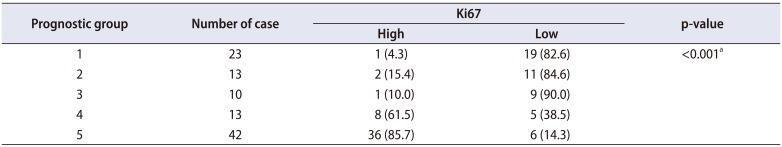
Of 101 cases, 98 cases (97.0%) were positive for Ki67 and 3 cases (3.0%) were negative for Ki67. As shown in
Table 2, among the proliferating cells, 50 of 98 cases showed a low Ki67 proliferation rate (less than 10%) and 48 of 98 cases showed a high Ki67 proliferation rate (10% or more). The Ki67 proliferation rate was evaluated and correlated with prognostic Gleason group as shown in
Table 2. A high Ki67 proliferation rate was seen in the higher prognostic groups (groups 4 and 5), whereas a low Ki67 proliferation rate or negative staining was found in the lower prognostic group (
Fig. 5). This was statistically significant (p<0.001, Fisher's exact test;
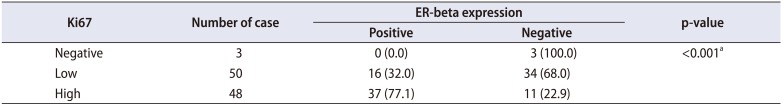
Table 2). The correlation between the Ki67 proliferation rate and ER-beta expression showed that 77.1% of cases with a high Ki67 rate were positive for ER-beta expression, whereas 68.0% of cases with a low Ki67 proliferation rate and 100% of cases with negative Ki67 proliferation were negative for ER-beta expression (
Table 3). Statistical analysis using Fisher's exact test showed a significant correlation between ER-beta expression and a high proliferation rate (p<0.001).
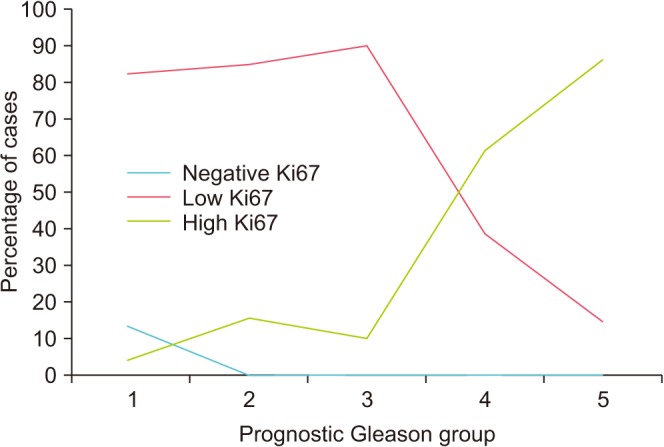 | Fig. 5Pattern of Ki67 proliferation rate in relation to prognostic group.
|
Table 2
Ki67 proliferation rate in relation to prognostic Gleason group
|
Prognostic group |
Number of case |
Ki67 |
p-value |
|
High |
Low |
|
1 |
23 |
1 (4.3) |
19 (82.6) |
<0.001a
|
|
2 |
13 |
2 (15.4) |
11 (84.6) |
|
|
3 |
10 |
1 (10.0) |
9 (90.0) |
|
|
4 |
13 |
8 (61.5) |
5 (38.5) |
|
|
5 |
42 |
36 (85.7) |
6 (14.3) |
|

Table 3
Correlation between Ki67 proliferation rate and ER-beta expression
|
Ki67 |
Number of case |
ER-beta expression |
p-value |
|
Positive |
Negative |
|
Negative |
3 |
0 (0.0) |
3 (100.0) |
<0.001a
|
|
Low |
50 |
16 (32.0) |
34 (68.0) |
|
|
High |
48 |
37 (77.1) |
11 (22.9) |
|

A total of 20 cases from various prognostic groups were evaluated by double-immunofluorescent staining for ER-beta and Ki67. Among the cases evaluated, 14 of 20 cases showed positive co-expression of ER-beta and Ki67 within the same tumor (
Fig. 6). In a few cases that co-expressed both markers, some of the proliferating cells were, however, negative for ER-beta (
Fig. 2). In 6 of 20 cases, the tumor cells did not co-express ER-beta and Ki67, showing that the two markers are mutually exclusive (
Fig. 6).
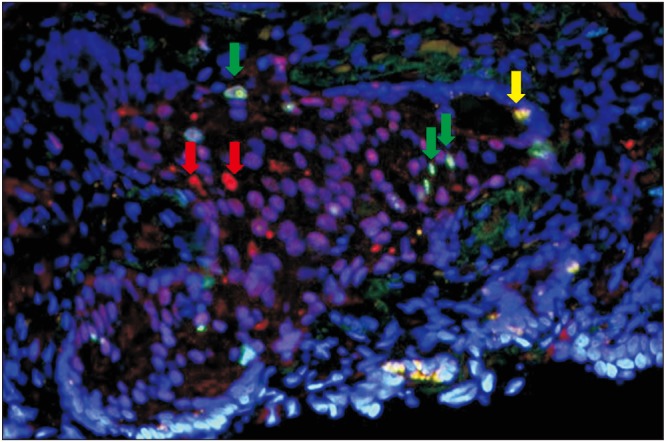 | Fig. 6Double-immunofluorescent staining (×40) showing cells with positive co-expression for estrogen receptor (ER)-beta and Ki67 stained yellow (yellow arrow) while some proliferating cells not expressing ER-beta are stained green (green arrows) and some ER-beta positive cells that are not proliferating are stained red (red arrows).
|
Go to :

DISCUSSION
We found that ER-beta was mainly expressed in cases with a high Gleason score (scores of 9 and above). Our findings showed a gradual increment in the number of positive cases with a Gleason score of 4 to 8 and an exponential increase in positivity for cases with Gleason scores of 9 and 10. This is in contrast with the findings of Asgari and Morakabati [
5] in which there was reduced expression of ER-beta in higher-grade prostatic adenocarcinoma compared with low- and intermediate-grade cancer. We have no obvious explanation for the inverse expression of ER-beta and Gleason score, but we can postulate that inadequate antibody specificity, ineffective antigen-bearing tissue retrieval, or the presence of unknown isoforms of ER protein may have affected the quality of immunohistochemistry [
5]. Besides, it was previously described that ER-beta, as detected by PPG5/10 antibody, may have a role in the process of de-differentiation of prostate adenocarcinoma, with a higher level present in less differentiated tumor [
13]. Our findings support the potential of ER-beta as a cancer-promoting agent as proposed by Christoforou et al. [
8] in 2014.
It was previously shown that the expression of ER-beta is more intense in PCa than in benign prostatic hyperplasia [
14]. Although we did not compare the staining intensity between benign and malignant prostate tissue, our own observation showed that the intensity of ER-beta staining is not restricted to the Gleason score.
The proliferation rate was high in cases with a Gleason score of 8 and above. Among those with positive Ki67 staining, the cases in prognostic groups 4 and 5 (Gleason scores of 8, 9, and 10) showed a higher percentage of proliferation rate than did the lower prognostic Gleason group. These findings agree with previous studies by Cowen et al. [
11] and Sulik et al. [
12] that reported that Ki67 levels were significantly higher in tumors with a high Gleason score. A study by Fixemer et al. [
15] concluded that ER-beta is expressed in the secretory epithelium of the prostate and possibly reflects androgen-dependent cancer cells and might have a chemo-preventive effect. However, our study focused on prostate adenocarcinoma alone, not normal and premalignant tissue.
The correlation between the Ki67 proliferation rate and ER-beta expression showed that those cases with high Ki67 proliferation were positive for ER-beta expression and those cases that were negative for Ki67 were negative for ER-beta expression. These findings indicate that proliferating prostate adenocarcinoma cells are expressing ER-beta. This observation was confirmed with double-immunofluorescent staining in which the majority of cases (14/20) showed co-expression of the markers at the individual cell level. However, in a smaller number of cases, co-expression was lacking, showing that ER-beta is expressed in individual cells independent of the proliferation marker. Whether the latter group (ER-beta positive, Ki67 negative) had a better prognosis remains to be determined.
Limitations
The use of a control group is important to avoid observational bias during the interpretation of data. However, financial constraints were the main culprit leading to the exclusion of a control group in this study. In the future, we will try to improve on this issue with a subsequent study or a continuation of this study.
Go to :

CONCLUSIONS
Positive ER-beta expression and a high Ki67 proliferation rate was associated with a high prognostic group. ER-beta and Ki67 are independent markers within tumor cells; hence, co-expression of ER-beta and Ki67 indicates a more aggressive tumor with a poorer prognosis and possibly cells that will respond to targeted therapy.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print









 XML Download
XML Download