This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
To investigate the effect on recurrence of vaporization of the tumor surroundings and suspicious areas with a plasma-kinetic (PK) system after transurethral resection (TUR) of nonmuscle invasive bladder cancer.
Materials and Methods
The study included 121 patients with a primary superficial bladder tumor who were randomized as those who underwent TUR with the PK system (Group 1, n=62) and those who underwent TUR with the monopolar system (Group 2, n=59). The vaporization procedure was performed by suppressing the cutting option of the PK system for a period, which would accumulate energy sufficient to make swelling-waves on the mucosa very close to the area of the loop to be vaporized.
Results
A total of 121 patients who met the study criteria were included for evaluation. Recurrence was determined in 21 patients in Group 1 (33.87%) and in 29 patients in Group 2 (49.15%) (p=0.088). Recurrence was close to the old resection site in 6 of 21 patients in Group 1, and in 13 patients in Group 2 (p=0.028); the difference was statistically significant. No statistically significant difference was determined between the two groups with respect to age, gender, number of tumor foci, rate or range of additional treatments applied, cigarette smoking rate, repeat TUR rate and rate of tumor en- countered in repeat TUR, T-stage, and tumor grade.
Conclusions
The effect of vaporization on recurrence by the PK system may seem similar to the effect of standard TUR, the recurrence- lowering effect surrounding nonmuscle invasive bladder cancers is better.
Go to :

Keywords: Recurrence, Surgery, cystoscopic, Urinary bladder neoplasms, Vaporization
INTRODUCTION
Just as in the world in general, bladder cancer is a common cancer in Turkey. It is the 2nd most common tumor among urogenital system tumors. The standard treatment for nonmuscle invasive bladder cancers is transurethral resection of bladder tumor (TURBT), and despite secondary treatments, such as intracavitary chemotherapy or immunotherapy, these tumors are frequently characterized by recurrence. Furthermore, bladder cancer is the most frequently diagnosed cancer with progression of stage and grade, treatment is one of the most expensive per patient, and bladder cancer is ranked 14th among causes of cancer-related deaths globally [
12].
Smoking has been identified as the most significant and widespread risk factor and the main cause in the etiology of bladder tumors [
3]. It is likely that there is a continuation of this effect during recurrence. Although the overall survival rate from nonmuscle invasive bladder cancers (
Fig. 1) is good, the heterogeneity of the cancer affects progression and recurrence rates in patients [
4]. Tumor grade is the most important parameter in determining recurrence and progression and in predicting prognosis. For example, while the progression rate of Ta tumors in long-term follow-up has been reported as 6%, the progression of high-grade tumors has been found to be 17% [
5].
 | Fig. 1Nonmuscle invasive bladder cancer.
|
Nonmuscle invasive bladder cancers showing highly variable recurrence rates are associated with tumor type and factors related to the location [
67]. In early recurrence, there is consensus that there is residual tumor presence after resection or that recurrence has occurred with the development of microscopic lesions that have been overlooked and not diagnosed [
891011]. Visual inspection with white light cystoscopy is not very reliable even for papillary tumors. Flat lesions, carcinoma
in situ (CIS), dysplasia, multifocal development, and microscopic lesions may be overlooked or insufficiently resected [
12].
In a study examining whether survival rates worsened with increasing recurrences, 10-year cancer-specific mortality rates for tumors showing 1, 2, 3, and 4 or more recurrences were found to be 6.9%, 9.7%, 13.7%, and 15.7%, respectively [
13]. The aim of treatment of these tumors with so much recurrence is to apply definitive treatment before muscular invasion starts [
14]. Patients for whom definitive treatment is delayed, that is, patients who receive less effective intravesical treatment, have poorer survival rates [
151617].
Several strategies have been developed with the aim of being able to make an early diagnosis and perform interventions for tumor recurrence. These include repeat transurethral resection (TUR) interventions, chemoimmunotherapies as additional treatments to resection, chemohyperthermia applications targeting deeper layers of the epithelium, frequent cystoscopic checks and cytological examinations, prevention of recurrence with resection rendering previously unseen foci visible by using photodynamic treatment options, quitting smoking, and lifestyle changes (e.g., change of occupation or residence).
Notwithstanding the contribution of these strategies, CIS foci can remain in close proximity to the main tumor. Thus, the aim of this study was to investigate the benefit of vaporization of the area surrounding the tumor with the target of supporting resection without the need for wider resection, which has the disadvantage of wider tumor seed areas.
Go to :

MATERIALS AND METHODS
This prospective study was planned in the context of a Scientific Research Project at Izmir Katip Çelebi University Medical Faculty Hospital. Approval for the study was obtained from the İzmir Katip Çelebi University Local Ethics Committee (approval number: 155) and informed consent was obtained from each patient. Patients were informed about the concerns of their case. The choice of methodology was not imposed but was left to the patient. The study included 121 patients diagnosed with primary nonmuscle invasive bladder cancer between January 2014 and June 2017 who were randomized and divided into two groups. Flipping a coin was used for randomization. The two groups were as follows: those who underwent TUR with the plasma-kinetic (PK) system (Group 1, n=62) and those who underwent TUR with the monopolar system (Group 2, n=59). Criteria for inclusion in the study were (i) primary nonmuscle invasive bladder cancers with a maximum of three foci and diameter <3 cm, (ii) regular follow-up for 2 years following resection, and (iii) similar criteria and applications of treatment in addition to postoperative single-dose intracavitary chemotherapy in both groups.
For each patient a record was made of age, gender, number of tumor foci, rate and type of additional treatments applied, smoking status, repeat TUR rates and rate of tumor encountered in repeat TUR, T-stage, and tumor grade.
Exclusion criteria were cases with squamous cell cancer, adenocancer, recurrent cancer, or metastatic bladder tumors; patients who had indications for additional treatment but rejected the treatment; or cases who did not complete treatment.
The bladder tumor areas were mapped on a bladder template. In cases with bladder lateral wall tumors, an obturator block was applied by the anesthetist before resection. The tumors were appropriately resected. In Group 1 patients, the vaporization procedure was completed by suppressing the cutting option of the PK system for a period to accumulate energy sufficient to make swelling-waves on the mucosa while closing off the loop to the vaporization area and from the edge of the resection outwards until a halo of approximately 2 cm was formed. Suspicious areas were also vaporized in a manner to include the whole inside of the area. As a result, an area of 5 to 6 cm in diameter was affected by the procedure (resection+vaporization). To understand the histopathological changes in the vaporized areas, resection biopsy was performed on this area in several cases. But the process was difficult in localized tumors near the urethral orifice, near the bladder neck, and on the anterior wall of the bladder. If the tumor was very close to the orifice, vaporization was not performed.
Statistical analysis was performed with IBM SPSS Statistics ver. 22 (IBM Co., Armonk, NY, USA) software. Values were described as numbers, percentages, means, and standard deviations. If the data were distributed normally, we used the Student's t-test and chi-square test; Fisher's exact test was used to compare means and categorical data. A p-value <0.05 was accepted as statistically significant.
Go to :

RESULTS
A total of 121 patients who met the study criteria were included for evaluation. Recurrence was determined in 21 patients from Group 1 (33.87%) and in 29 patients from Group 2 (49.15%), but this difference was not statistically significant (p=0.088). In Group 1, the recurrence was close to the old resection site (within a 2-cm vaporization area) in 6 of the 21 patients. In Group 2, the recurrence was within the old resection site or within 2 cm in 13 cases (p=0.028). The reduction in recurrence in the vaporized area was at a statistically significant level.
No statistically significant difference was determined between the two groups with respect to age, gender, number of tumor foci, follow-up period, rate and range of additional treatments applied (additional treatment was applied to 26 patients in Group 1 and to 25 patients in Group 2), cigarette smoking rate (% and packs/year), repeat TUR rate and rate of tumors encountered in repeat TUR (Group 1, 3; Group 2, 9), T-stage, and tumor grade. The findings are shown in
Tables 1,
2.
Table 1
The demographic characteristics of the patients in both groups
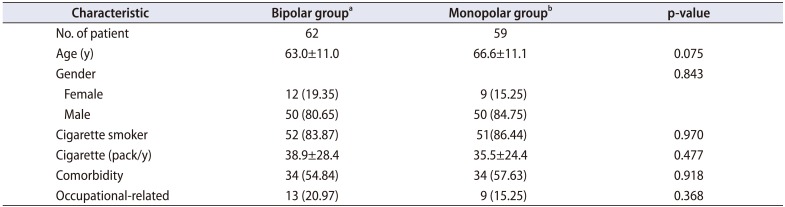
|
Characteristic |
Bipolar groupa
|
Monopolar groupb
|
p-value |
|
No. of patient |
62 |
59 |
|
|
Age (y) |
63.0±11.0 |
66.6±11.1 |
0.075 |
|
Gender |
|
|
0.843 |
|
Female |
12 (19.35) |
9 (15.25) |
|
Male |
50 (80.65) |
50 (84.75) |
|
Cigarette smoker |
52 (83.87) |
51(86.44) |
0.970 |
|
Cigarette (pack/y) |
38.9±28.4 |
35.5±24.4 |
0.477 |
|
Comorbidity |
34 (54.84) |
34 (57.63) |
0.918 |
|
Occupational-related |
13 (20.97) |
9 (15.25) |
0.368 |

Table 2
Operative and postoperative data
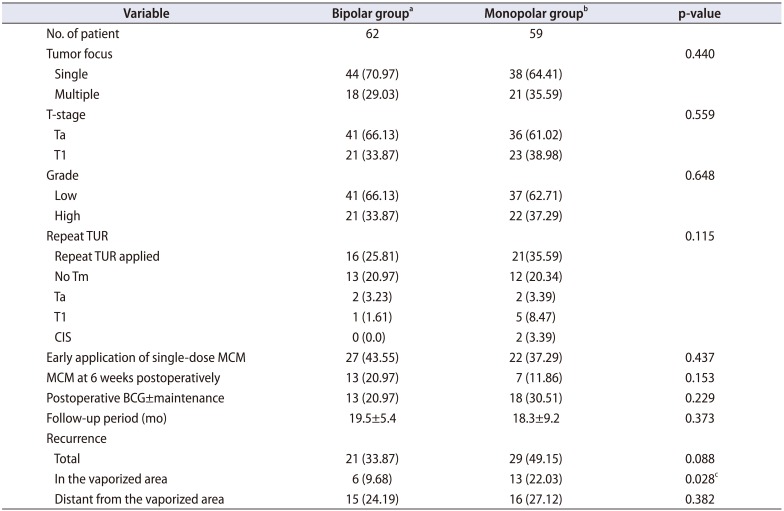
|
Variable |
Bipolar groupa
|
Monopolar groupb
|
p-value |
|
No. of patient |
62 |
59 |
|
|
Tumor focus |
|
|
0.440 |
|
Single |
44 (70.97) |
38 (64.41) |
|
Multiple |
18 (29.03) |
21 (35.59) |
|
T-stage |
|
|
0.559 |
|
Ta |
41 (66.13) |
36 (61.02) |
|
T1 |
21 (33.87) |
23 (38.98) |
|
Grade |
|
|
0.648 |
|
Low |
41 (66.13) |
37 (62.71) |
|
High |
21 (33.87) |
22 (37.29) |
|
Repeat TUR |
|
|
0.115 |
|
Repeat TUR applied |
16 (25.81) |
21(35.59) |
|
No Tm |
13 (20.97) |
12 (20.34) |
|
Ta |
2 (3.23) |
2 (3.39) |
|
T1 |
1 (1.61) |
5 (8.47) |
|
CIS |
0 (0.0) |
2 (3.39) |
|
Early application of single-dose MCM |
27 (43.55) |
22 (37.29) |
0.437 |
|
MCM at 6 weeks postoperatively |
13 (20.97) |
7 (11.86) |
0.153 |
|
Postoperative BCG±maintenance |
13 (20.97) |
18 (30.51) |
0.229 |
|
Follow-up period (mo) |
19.5±5.4 |
18.3±9.2 |
0.373 |
|
Recurrence |
|
|
|
|
Total |
21 (33.87) |
29 (49.15) |
0.088 |
|
In the vaporized area |
6 (9.68) |
13 (22.03) |
0.028c
|
|
Distant from the vaporized area |
15 (24.19) |
16 (27.12) |
0.382 |

Patients who could not tolerate the postoperative single-dose intracavitary treatment (a single dose could not be applied or the chemotherapeutic agent could not be held for a sufficient time in the bladder because of tenesmus) and those who were compliant with the treatment are shown in
Table 2.
As a result of the randomized resection biopsies obtained from vaporized areas, necrotic areas extending as far as the muscle were reported (
Figs. 2,
3).
 | Fig. 2Vaporization following resection, showing necrosis of the epithelium and muscle tissue (H&E, ×100).
|
 | Fig. 3Histopathological image of a specimen taken from the same area following vaporization. Varying degrees of necrosis are observed: necrosis is generally more active in the mucosa, the epithelium has fallen away in all areas, and evident necrosis is seen in the muscle layer in some samples (H&E, ×100).
|
Because resection was completed by the same method in both groups and vaporization was applied so as not to make contact with the mucosa, no difference was seen between the groups in terms of complications. In the resections of bladder lateral wall tumors, because resections were applied with obturator block in both groups even if there was a concern about obturator reflex, no significant problems (e.g., bladder rupture) occurred.
As a result of the resection and vaporization, an area of approximately 5 cm in diameter was cleaned of the tumor. The resection area was 1 to 2 cm in diameter and the surrounding halo formed was 2-cm wide; thus, the whole area was 5 to 6 cm in diameter (
Fig. 4).
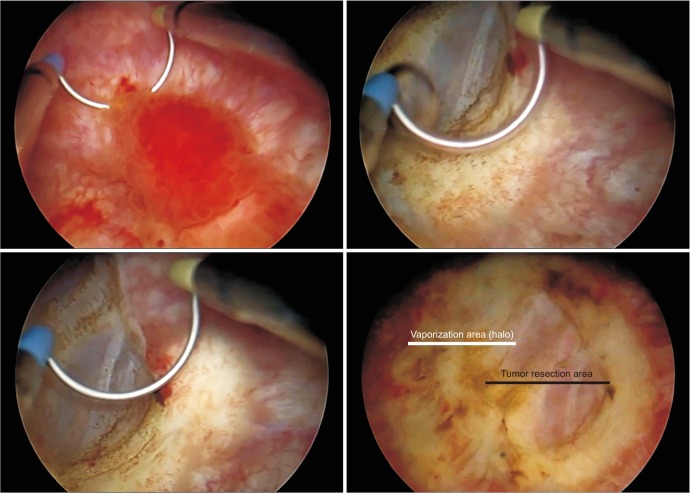 | Fig. 4Tumor resection and vaporization of the surrounding area (the vaporized area is seen in the form of a halo surrounding the resection area and paler than the mucosa).
|
Go to :

DISCUSSION
The initial treatment for Ta and T1 tumors is TURBT including the muscular layer [
1819]. This treatment is important in terms of both correct grading and the effect on subsequent approaches. If there is a suspicion of incomplete resection or that muscle tissue has not been removed, repeat resection must be applied (except in cases of TaG1 tumor and primary CIS). In T1 and/or G3 tumors, the second TURBT for re-staging should be applied 2 to 6 weeks after the first TUR (except for CIS alone) [
20].
The frequent recurrence of nonmuscle invasive bladder cancers causes problems such as the difficulty patients have accepting frequent follow-up examinations and repeated TURBT when necessary and an increased workload for the physician. The physician must explain to the patient that procedures have been applied correctly and it can be difficult for the patient to withstand. Although there has been much research on the subject of the treatment of residual tumors and the prevention of recurrence, the desired levels of success have not yet been obtained.
Bacillus Calmette-Guerin (BCG) treatment is the only immunotherapy that has been approved by the US Food and Drug Administration as a prophylaxis and for treatment of bladder cancers. Despite the success of this treatment, recurrence rates remain high and progression is still possible [
21]. The duration of contact with the bladder is very important and urothelial tumors are widely accepted as sensitive to immunotherapy. Therefore, new treatment modalities are based on immune response (such as cancer vaccinations and subcomponents of BCG and checkpoint inhibitors). As a characteristic of the bladder epithelial structure, umbrella cells on the surface express several different glycosaminoglycans. These adopt a role as a barrier against toxic substances [
22]. These hydrophilic molecules immediately spread over the cell surface and form a barrier between the urothelium and the bladder cavity. Therefore, chemotherapeutic agents, including immunotherapeutic agents, often do not spread to the deep cell layer and are not effective [
23].
With the initiation of use of cell wall components such as BCG products, toxicity decreased and the effect of greater adherence to the urothelium increased.
Mycobacterium phlei cell wall extract is not pathological in humans and has been used in CIS cases in particular [
24].
It is well known that greater penetration of chemotherapeutic drugs can be provided with application of hyperthermia. When mitomycin C (MMC) and epirubicin are applied using a 915 microwave intravesical applicator to patients with previously unsuccessful treatment, a 2-year disease-free interval was achieved at a rate of 47%. A 10-year progression-free survival was obtained in patients receiving MMC with thermotherapy compared to MMC treatment alone [
25].
With respect to photodynamic diagnosis (PDD), good studies have been conducted related to the importance of photodynamic resection and it was determined that highly significant benefits were provided in the prevention of recurrence in a 9- to 12-month follow-up period, but it was not found to be significant for progression [
12].
CIS is a high-grade tumor of flat, nonpapillary configuration surrounded by the urothelium. They may be seen as a reddish, mild swelling from the mucosa, but usually they cannot be seen. The majority are diagnosed with biopsy [
26]. The results of several studies reported that there was at least a 15% to 40% chance of finding residual tumors during standardized delayed second TUR surrounding the initial tumor bed after the initial TURBT [
27]. The identification of these areas where resection (or vaporization) has been applied or is to be applied also means that there will be much less development of recurrence. Therefore, the possibility of vaporization of the area surrounding the tumor can be considered beneficial in the treatment of these foci.
Rather than applying resection under blue light to the CIS foci or to small papillary structures, which creates new seeding areas in terms of recurrence risk, vaporization can be applied using PK energy. With vaporization, there is the possibility of repairing these areas which have undergone necrosis and deteriorate slowly with normal bladder mucosa epithelium. This technique was first introduced by a Romanian group in 2011 [
28], but the cutoff value of a 2-cm vaporization area was not identified for local recurrence by this group. We attempted to standardize this process.
High-risk patients, especially high-grade patients, may emerge as BCG-refractory even if BCG is given, and even if secondary BCG induction or another alternative therapy is applied (MCC, valrubicin, etc.), salvage cystectomy may often be necessary. Perhaps these patients should have been referred earlier for more aggressive treatment (e.g., early cystectomy), or by identifying most tumor foci in the bladder mucosa with PDD, they should have been treated with monopolar or preferably PK energy systems.
Our study had some limitations. First, we aimed to investigate the effect of vaporization of the tumor surroundings on recurrence, but although the recurrence rate around the tumor was less in the vaporization group, the total recurrence rate was not different. This situation can be overcome by a wider series of studies. Additionally, our work is continuing because it is promising, and we are about to achieve the expected result. Another limitation was that the follow-up period seemed to be short, but the follow-up of the patients continues. A limitation was that vaporization cannot be easily applied to the bladder neck and bladder wall near the urethral orifice. Moreover, it is difficult to use vaporization on the anterior wall mucosa of the bladder during TURBT, due to air bubbles at the anterior wall of the bladder. For this reason, vaporization was performed for anterior wall tumors by using manual suprapubic pressure and in the correct way towards the mass. Furthermore, we did not consider the time of recurrence. We did not consider it important to include the time of recurrence in the analysis; however, this may be perceived as a limitation.
In conclusion, in nonmuscle invasive bladder cancers, the initial and best treatment is good radical TUR and, when necessary, instillation [
26]. For radical TUR, it is necessary for the resection to be made with sufficient depth and width. At the same time, this expands tumor seeding areas. Therefore, rather than applying wide resections, the goal can be reached with vaporization. It can also be considered that there is the possibility that the vaporized area, which denudes slowly over time, can be covered with healthy mucosa. In our study we found that vaporization of the area surrounding the resection with a TUR PK system is effective and safe.
In the application of intravesical chemotherapy for treatment of bladder tumors, it has been shown that the effect of the treatment on cancer cells located more deeply than the superficial cell layer remains insufficient [
23]. In resection biopsies obtained from the vaporized areas, it was seen that the effect could be as deep as the muscle layer (
Figs. 2,
3). The main aim in the prevention of recurrence should be to remove potential tumor foci from the bladder mucosa by complete and accurate identification without causing serious damage to the mucosa of these areas. The current authors have an ongoing project to minimize recurrence by combining the PK energy TUR system with PDD.
Go to :

CONCLUSIONS
Vaporization of the area surrounding the resection with a TUR PK system is effective and safe. Although the effect on recurrence of vaporization with PKs may seem similar to that of standard TUR, the recurrence-lowering effect is better surrounding nonmuscle invasive bladder cancers. With the exception of locations such as the bladder anterior wall, which cannot easily be reached with a resectoscope, and ureter orifices, the vaporization area can be further expanded when the location to be resected is suitable. Our studies are continuing to further improve the success of this method and examine outcomes.
Go to :

In patients with nonmuscle invasive bladder cancer, the standard initial treatment includes a complete transurethral resection of bladder tumor (TURBT), examination under anesthesia, and a single postoperative instillation of intravesical chemotherapy in the appropriate patient [
1]. Although the transurethral resection may be complete, these patients remain at high risk for recurrent disease. For patients in whom the initial TURBT is consistent with nonmuscle invasive disease, patients are stratified for risk of recurrence (low, intermediate, high) based upon stage, grade, and the number and size of tumors. This stratification provides the basis for determining whether additional intravesical adjuvant therapy is indicated. Suspicion of incomplete resection or inadequate muscular layer removal, re-resection is warranted (except in cases of G1 and primary carcinoma
in situ [CIS]). In T1 and/or G3, the second TURBT for re-staging should be applied 2 to 6 weeks after the first TURBT (except for CIS alone) [
2]. Bacillus Calmette-Guerin treatment is the only immunotherapy that has been approved by the US Food and Drug Administration as prophylaxis and for treatment of bladder cancers, despite recurrence rate remains high [
3]. It is well known that greater penetration of chemotherapeutic drugs can be provided with application of hyperthermia. Photodynamic resection has been shown to benefit the prevention of recurrence. CIS of the bladder are high grade lesions of flat, non-papillary in morphology and are highly prone to recurrence. Previous studies have shown that 15% to 40% of post-TURBT patients have residual cancer in standardized delayed re-resection [
4]. Successful identification of these lesions likely will lower recurrence rate. Therefore, vaporization of the surrounding area of the cancer bed is considered to be beneficial.
Compared to re-resection, which may bring about new seeding tumors, vaporization using plasma-kinetic energy can repair necrotic areas and prevent seeding [
5].
Yılmaz et al. [
6] analyzed 121 patients with primary nonmuscle invasive bladder cancer between January 2014 and June 2017 and were randomized into TURBT with the plasma-kinetic system (n=62) and TURBT with the monopolar system (n=59). This study was performed to investigate the effect of vaporization on recurrence of the tumor surroundings and suspicious areas with a plasma-kinetic system following TURBT. The difference of recurrence rate was not statistically significant (p=0.088) between the two groups. It also showed that the recurrence reduction in the vaporized area was at a statistically significant level. Although this study was limited by the small sample size and not utilizing time-to-event analysis, it is noteworthy that the authors suggested the advantages of vaporization over wide resections when prevention of tumor seeding status post-TURBT is sought after, especially in areas surrounding the nonmuscle invasive bladder cancers, which was confirmed by Student t-test and chi-square, and Fisher's exact test.
In summary, the present study by Yılmaz et al. [
6] further adds validated results to the previous evidences that plasma-kinetic vaporization is an effective and safe method to prevent seeding of nonmuscle invasive bladder cancer. Thus, I believe that clinicians should now consider the use of vaporization along with standard monopolar/bipolar TURBT system in the management patients with nonmuscle invasive bladder cancer in real-world practice.








 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download