INTRODUCTION
The prevalence of atrial fibrillation (AF) at all ages is higher in men compared to women.
1) However, in general population, the absolute number of women who have AF was similar in men and women because of the longer life expectancy in women.
2) Several studies have investigated the gender differences in the epidemiology, presentation and prognosis of AF.
3)4)5) Women with AF are more likely to develop stroke and are at a higher risk of mortality than men.
6)7) AF was also related with cardiovascular mortality, especially more in women.
8) Large population cohort study showed that AF is a much greater risk factor of cardiovascular death in women about twice.
9) AF and mortality were shown to be associated with an odds ratio for death of 1.5 in men and 1.9 in women according to Framingham cohort study.
6) In addition, AF in women is known to be associated with longer episode, higher ventricular rates and impaired quality of life.
4)10)11) Therefore, maintenance of sinus rhythm (SR) can be more helpful to women. Radiofrequency catheter ablation (RFCA) for rhythm control appears to be an effective alternative in women with drug refractory AF.
Recent studies demonstrated women had controversial outcome of rhythm control by drug or RFCA compared to men.
12)13)14) The known main cause of disparity is not related to hormone but social characteristics of woman. Woman or physician to treat woman often prefer medical treatment than invasive procedure including catheter ablation. So undertreated period is usually longer in women than men.
13)15) Time lag from diagnosis to RFCA for AF may attenuate the effectiveness of the procedure. Therefore, pure gender-related difference is difficult to be confirmed due to referral bias. We hypothesized that clinical long-term outcome after RFCA for AF was different between women and men with similar AF history.
METHODS
Study population
A total of 1,875 patients underwent 2,307 consecutive RFCA for AF at Korea University Anam Hospital between June 1998 and May 2014 (
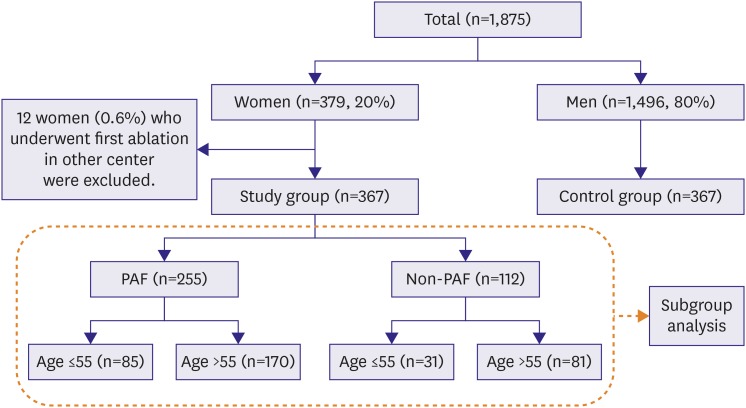
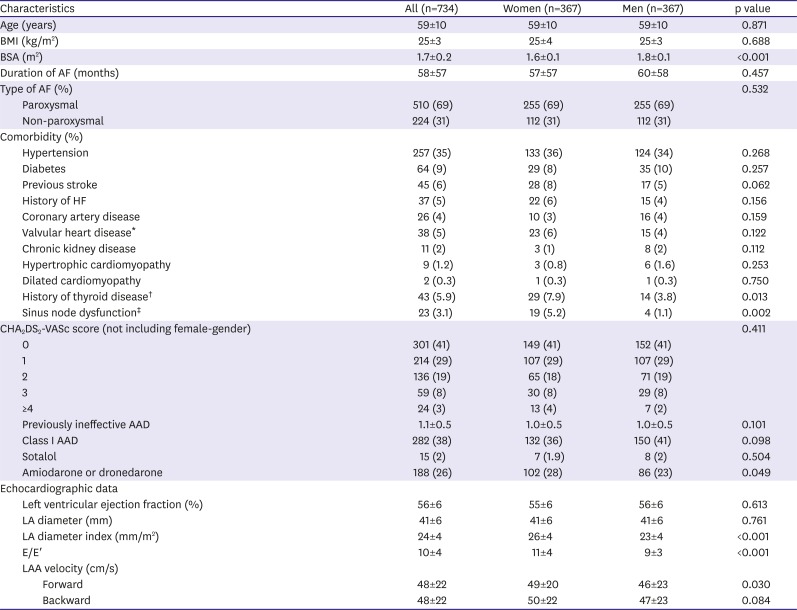
Figure 1). Patients with highly symptomatic drug refractory AF were selected for ablation. Among them, 379 patients (20.2%) were women, and 367 patients (19.5%) who underwent first ablation were included in this study. Twelve patients who underwent first ablation in other hospital were excluded. Three hundred sixty-seven men were selected as control to 1:1 match the study group by age (±1 year), type of AF (paroxysmal or non-paroxysmal), and duration of AF before index procedure (±2 years). We considered index procedure era as matching criteria to decrease bias related with ablation technology development.
Figure 1
Schematic representation of study population as the gender, type of AF, and age. The number of patients in each category and their proportional representation are shown. Clinical outcomes after ablation of subgroups in dotted line were analyzed.
AF = atrial fibrillation; PAF = paroxysmal atrial fibrillation.
Definition of variables and outcome
Duration of AF was calculated by the period between the time of the first diagnosis and index procedure. It was assessed by confirming previous medical record. Definition of clinical type of AF (paroxysmal or non-paroxysmal) was followed the American Heart Association (AHA)/American College of Cardiology (ACC) recommendations.
16) Atrial arrhythmia recurrence was defined with documentation of atrial arrhythmia (>30 seconds) including AF or atrial tachycardia (AT). However, we did not consider atrial arrhythmia within blanking period as recurrence. Blanking period was defined as 3 months after procedure. Procedural success was no atrial arrhythmia recurrence during follow-up period with or without anti-arrhythmic drug (AAD). Procedure-related (within 1 week) complication were defined as hematoma requiring transfusion, arteriovenous fistula requiring operation, dissection of major vessel, periprocedural cerebrovascular accident (CVA), tamponade, shock or death, etc. Primary outcome is atrial arrhythmia recurrence. Secondary outcome is thromboembolic event.
Peri-procedural management and ablation strategy for AF
AADs were discontinued in more than 5 half-lives and amiodarone was discontinued at 1 month prior to procedure. Anticoagulation was continued for at least 2 months after ablation in all patients regardless of CHA2DS2-VASc score. Transesophageal echocardiography was performed in all patients within 24 hours immediately before procedure to rule out the presence of left atrial (LA) thrombi. All the patients underwent electrophysiology study and ablation after written informed consent was obtained.
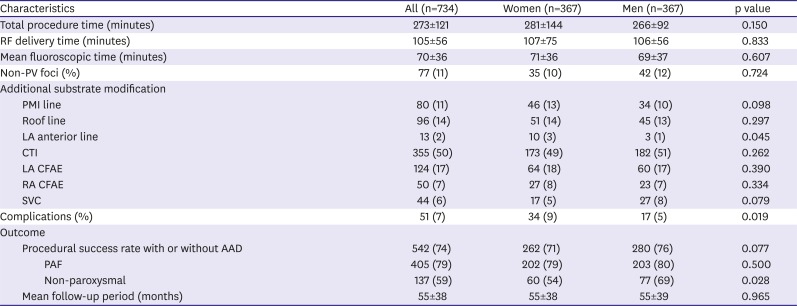
Our ablation procedure has evolved over the 15-year period of this study. We performed focal ablation at the pulmonary vein (PV) guided by Lasso catheter (Biosense Webster, Inc., Irvine, CA, USA) from 1998 to 2004. Non-contact mapping-guided PV ablation since 2005. We have started to perform circumferential ablation of 4 PVs guided by NavX (St. Jude Medical Inc., St. Paul, MN, USA) since 2007. The end point of PV isolation (PVI) was elimination of PV potentials on the 10-bipole ring-shaped catheter at each PV. Since 2009, subsequent ablation strategy differed according to the clinical type of AF. In case of patient with paroxysmal AF (PAF), non-PV triggers of sustained AF were evaluated and targeted after PVI. To identify triggers initiating AF, multiple electrical cardioversion was performed subsequently under isoproterenol (10–20 µg/min) infusion. Triggers were evaluated if they caused re-initiation of sustained or non-sustained AF within 2 minutes after repetitive cardioversion. After PVI, same protocol was repeated at least 3 times, to identify non-PV foci initiating AF. In case of patient with non-PAF, additional substrate modification was performed after PVI. If AF was sustained after PVI, 3-dimensional complex fractionated atrial electrograms (CFAEs) of LA were mapped during 6 seconds of AF with NavX software. Ablation was performed using the CFAE map until AF termination or organized to an AT, or the fractionated potential in LA was eliminated. The CFAE in the right atrial (RA) was targeted for ablation if AF persisted after LA CFAE ablation. If AF was organized to AT during PVI or CFAEs guided ablation, activation and entrainment mapping guided ablation were performed. If AF/AT was terminated by ablation, inducibility of sustained AF/AT by pacing was evaluated in patients with non-PAF. If SR is not restored after ablation as mentioned above, patient was cardioverted with internal or external direct-current electrical cardioversion. The endpoint of procedure for PAF was to eliminate all triggers including PV. Endpoint of procedure for non-PAF was termination of AF/AT and no induction of sustained atrial arrhythmia by pacing protocol.
Follow-up
Every patient visited outpatient clinics 2 weeks after discharge and every 2–4 weeks during the initial 3 months to monitor the occurrence of complications and early recurrence. Patient visited every 3 months after the blanking period. Electrocardiography was routinely checked during each follow-up at outpatient clinics. 24-hours Holter monitoring or 7-days event recorder was performed at 1, 3, 6, 9, and 12 and then every 6 months after discharge in asymptomatic patients. In case of patients with implanted intracardiac device, the recurrence was checked by interrogation of device. If patients experienced tachycardia-related symptom without documentation, it was confirmed by additional Holter or event recorder. During blanking period, prescription of AADs was at the discretion of physicians. Repeat ablation for recurred AF/AT was not allowed during the blanking period. AAD was discontinued after the end time of the blanking period.
Subgroup analysis
Subgroup analysis was performed in categories defined by clinical AF type and age. We set up criteria of age as 55 to evaluate hormonal effect. It has been known that mean age at natural menopause in Asian woman is 49 years and hormone did not affect human body at age over than 55.
17) Clinical outcome was estimated in each 4 subgroups.
Statistical analysis
Continuous variables are expressed as a mean standard deviation. Categorical variables are reported as counts with percentage. An independent Student's t-test was used to compare continuous variables between women and men groups. Categorical variables were compared by a χ2 test or Fisher's exact test. Time to AF/AT recurrence was calculated using the Kaplan-Meier analysis with comparisons between both genders performed by log-rank statistics. To analyze independent predictors of AF recurrence after ablation and clinical outcomes, multivariable Cox regression model was used. Confounders adjusted in the multivariable model included age, sex, LA diameter index, and history of heart failure (HF). The hazard ratio (HR) and 95% confidence interval (CI) were presented in the results. A p value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS 20.0 software package (SPSS Inc., Chicago, IL, USA).
DISCUSSION
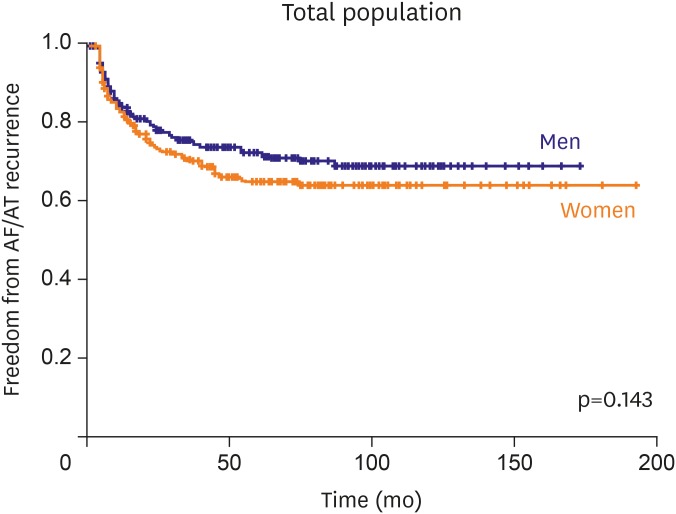
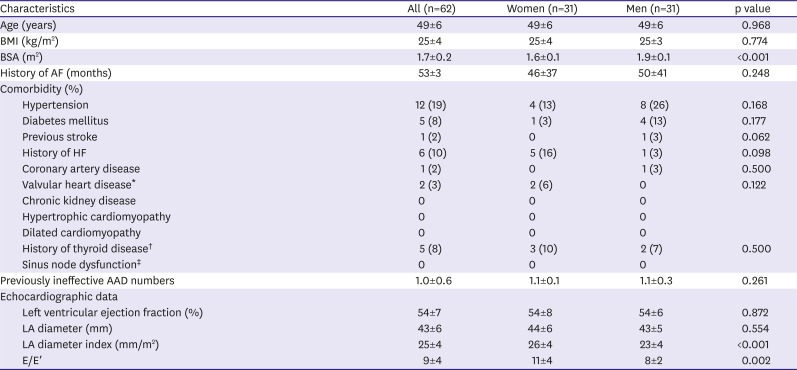
In this study, we found that 1) women had higher LAD index and E/E′ ratio compared to men with matched history of AF and similar number of failed AADs; 2) clinical outcome after single procedure did not differ between both gender groups; and 3) women with non-PAF, especially in age ≤55 years has worse outcome compared to men. Many previous papers concluded that gender difference of clinical outcome after RFCA for AF was related with referral bias. We ruled out impact of referral bias by matching to presented pure gender difference. We selected men group as control to 1:1 match the study group by age, type of AF, history of AF, and index procedure era.
Women are more likely to experience more symptomatic episodes, significantly higher ventricular rates and impaired quality of life.
10)11) Women with AF have worse outcome compared to men for some reason. Stroke Prevention in Atrial Fibrillation (SPAF) trial showed female gender was independent predictor of stroke.
18) Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) trial demonstrated significantly higher risk of thromboembolism in women.
7) AF was also related with cardiovascular mortality, especially more in women. The risk of death in non-valvular AF in the Framingham cohort showed that AF was associated with an odds ratio for death of 1.5 in men and 1.9 in women.
8) The Copenhagen City Heart Study as large population cohort study showed that AF was a much more pronounced risk factor for cardiovascular death in women about twice.
9)19) As this result, woman with AF suffers from worse prognosis than man.
Several papers showed controversial result about gender-related impact to clinical outcome after RFCA for AF. Forleo et al.
13) demonstrated that clinical outcome did not differ even though women were older with significantly higher LA diameter. The ratio of freedom from atrial arrhythmia recurrence was 82.7% in men and 83.1% in women during mean follow-up of 23 months. Patel et al.
15) evaluated clinical outcome and complication of referred 3,265 women who underwent PV antrum isolation. Referred women were older and had a higher prevalence of stroke and hypertension than men. After 24 months of mean follow-up, women had lower success rates than men (68.5% vs. 77.5%). Forleo et al.
13) and Patel et al.
15) reported the longer untreated period, more failed AAD pre-ablation in women group. This referral bias impacted the results. We included all referred woman patients who underwent first ablation in single center in this study. However, men were selected as control to 1:1 match with women by above mentioned criteria to minimize referral bias or undertreated period gap. As a result, numbers of failed AAD, CHA
2DS
2-VASc score and most of comorbidity between both groups were similar. Procedure or ablation time were not different. Finally, long-term outcome after single procedure was not different between both groups.
We divided the consecutive population into 4 subgroups based on clinical type of AF and age, in order to analyze the effects of hormone on the outcome. Clinical outcome discrepancy between both genders is evident in subgroup younger than 55 years or subgroup with non-PAF. Characteristics related to RFCA in women have been reported as small vessel size, more epicardial fat, higher peri-procedural complication rate. The results of our study cannot be explained even considering these factors.
In this study, women had higher rate of cardiac tamponade and puncture related complication compared to men as shown. Such complications may influence procedural acute success and outcome. Other studies reported women had higher risk for developing cardiac tamponade.
20)21)22) The mechanism was not obvious yet. Small body surface area (BSA), lower LA volume, thinner LA wall, and higher incidence of atrial septal aneurysm in women were suggested as cause.
23)24) In this study, measured LA size was not different between both groups but other cause was not explored. The difference of complication rate between both genders was not observed in each subgroup.
The gender difference in diastolic function of the heart might be related to these results. HF with preserved EF is more prevalent in women than men, but the basis for this difference remains unclear. Incidences of arterial stiffness and concentric left ventricular remodeling were associated with the development of HF in women greater than in men despite the preserved EF.
25) This study did not show any differences in comorbidity, whereas E/E′ was observed to be significantly higher in women. However, this gender difference is associated with cardiovascular aging. As aging is known to decrease the diastolic heart function in women, it is difficult to explain the difference in prognosis in those under the age of 55 with non-PAF.
Some studies have found gender-related difference in arrhythmia impacted by hormone.
26)27)28) Gender differences in the subgroup with non-PAF were more pronounced than in the subgroup with PAF. This is thought to be related to the substrate formation as a mechanism to sustain AF. It was the same in non-PAF group of less than 55 years that had shown a large difference of outcome. LA diameter index was greater in women despite the period since diagnosis was the same. Mechanism of the difference in the substrate formation between men and women is still unclear. Some studies noted the role of estrogen. Estrogen in menopausal women raises the risk of AF. There are experimental research that the estrogen affects aspect of the cardiac arrhythmia or cardiac electricity profile.
27)28) However causation is not determined yet and still debatable. It seems further studies will be required on the relationship between estrogen and the substrate.
In many cardiovascular diseases, female gender is known to have a protective role. In the similar age, men show significantly higher prevalence of AF compared to women. It is questionable that the result of this study can impact on the selection of candidates for catheter ablation. Catheter ablation had already confirmed the effectiveness of the treatment for drug refractory symptomatic AF by many prior studies.
29)30) Our study had shown the catheter ablation was effective in women who are 56 years old or older, particularly in those with PAF as same as in men. Catheter ablation is good choice to create a better outcome if the medical treatment is not effective to such patients. The efficacy of the procedure is higher and the procedure related complications was lower in younger age. Therefore, it is necessary to improve the long-term prognosis by ablation. However, decision for ablation should be carefully considered to young women, especially if that etiology is not clear. In these patients, prevalence of AF is low compared to men except for valvular AF.
AF in these patients has a possibility related with genetic variant or myocardial disease. Before making the decision for the procedure in young women with AF, the evaluation of cause should be considered.
The retrospective design was main limitation of the study. Although the matching was performed to reduce referral bias but the potential for selection bias still exists. In order to reduce such bias, all women were included in the study subjects who had undergone ablation without screening of the investigator. In order to select the man matching at the ratio of 1:1, first, all men who were suitable to the criteria per one woman were sorted. Then, randomly matching is by a computer. This method was possible as men were 4–5 times more than women patients. This study was focused on the outcome of RFCA for AF, not the overall gender difference in AF. The mechanism of gender difference in AF incidence might be different from the outcome of RFCA. There is also the uncertainty of the AF duration. Because the criteria for AF duration were set to the time of diagnosis, there may be a difference in period from the prior symptom onset year of AF.
The reasons for the long-undertreated period of women are thought to be 2. First, it is the preference of medication therapy after diagnosed rather than procedural treatment, and the secondly, it is that delayed diagnosis by being mistaken the symptoms as pre-diagnostic stress, emotional problems or menopausal symptoms. The first reason can be reflected for the applied AF duration in this paper, but it is difficult to reflect in the second reason. Since the exact duration of AF is impossible to measure based on the beginning of the symptoms, the AF duration was calculated on the basis of the diagnosis.
In respect to the treatment group selection, since the patients from long time ago were included so that it was possible to show differences from the current profile where the procedural experiences had been increased with technological advancement. Prior to 2004, most of the patients were with PAF and it is considered to have no significant impact on the difference from non-PAF Patients were classified to subgroup by age to observe the effects of hormone. However, such classification had great individual variation. In addition, the age was taken as record at the time to receive a procedure, but the arrhythmia substrate has been formed for relatively long time, which implicates there is the higher the probability of mixed in the intermediate group.
The clinical outcome after RFCA was not different between both genders regardless of referral bias. However, the gender difference became evident in patients under 55 years with non-PAF.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download