1. Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996; 334:1084–1089. PMID:
8598866.
2. Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH. CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS). Circulation. 2000; 102:624–629. PMID:
10931801.
3. Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006; 354:1706–1717. PMID:
16531616.
4. Steinhubl SR, Berger PB, Mann JT 3rd, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002; 288:2411–2420. PMID:
12435254.
5. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Kardiol Pol. 2017; 75:1217–1299. PMID:
29251754.
6. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016; 134:e123–e155. PMID:
27026020.
7. Kim C, Shin DH, Ahn CM, et al. The use pattern and clinical impact of new antiplatelet agents including prasugrel and ticagrelor on 30-day outcomes after acute myocardial infarction in Korea: Korean Health Insurance Review and Assessment data. Korean Circ J. 2017; 47:888–897. PMID:
29035430.
8. Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005; 366:1607–1621. PMID:
16271642.
9. Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001; 345:494–502. PMID:
11519503.
10. Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005; 352:1179–1189. PMID:
15758000.
11. Hulot JS, Bura A, Villard E, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006; 108:2244–2247. PMID:
16772608.
12. Park KW, Kang J, Park JJ, et al. Amlodipine, clopidogrel and CYP3A5 genetic variability: effects on platelet reactivity and clinical outcomes after percutaneous coronary intervention. Heart. 2012; 98:1366–1372. PMID:
22735685.
13. Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010; 56:919–933. PMID:
20828644.
14. Park KW, Park JJ, Jeon KH, et al. Clinical predictors of high posttreatment platelet reactivity to clopidogrel in Koreans. Cardiovasc Ther. 2012; 30:5–11. PMID:
21129165.
15. Park KW, Kim HS. Options to overcome clopidogrel response variability. Circ J. 2012; 76:287–292. PMID:
22240602.
16. Xie X, Ma YT, Yang YN, et al. CYP2C19 phenotype, stent thrombosis, myocardial infarction, and mortality in patients with coronary stent placement in a Chinese population. PLoS One. 2013; 8:e59344. PMID:
23555019.
17. Jeong YH. “East asian paradox”: challenge for the current antiplatelet strategy of “one-guideline-fits-all races” in acute coronary syndrome. Curr Cardiol Rep. 2014; 16:485. PMID:
24668607.
18. Suh JW, Lee SP, Park KW, et al. Multicenter randomized trial evaluating the efficacy of cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease: results of the CILON-T (influence of CILostazol-based triple antiplatelet therapy ON ischemic complication after drug-eluting stenT implantation) trial. J Am Coll Cardiol. 2011; 57:280–289. PMID:
21232664.
19. Park KW, Jeon KH, Kang SH, et al. Clinical outcomes of high on-treatment platelet reactivity in Koreans receiving elective percutaneous coronary intervention (from results of the CROSS VERIFY study). Am J Cardiol. 2011; 108:1556–1563. PMID:
21880289.
20. Lee K, Yoo SY, Suh J, et al. Efficacy of cilostazol on inhibition of platelet aggregation, inflammation and myonecrosis in acute coronary syndrome patients undergoing percutaneous coronary intervention: The ACCEL-LOADING-ACS (ACCELerated inhibition of platelet aggregation, inflammation and myonecrosis by adjunctive cilostazol loading in patients with acute coronary syndrome) study. Int J Cardiol. 2015; 190:370–375. PMID:
25956540.
21. Angiolillo DJ, Shoemaker SB, Desai B, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation. 2007; 115:708–716. PMID:
17261652.
22. Park KW, Kang SH, Park JJ, et al. Adjunctive cilostazol versus double-dose clopidogrel after drug-eluting stent implantation: the HOST-ASSURE randomized trial (Harmonizing optimal strategy for treatment of coronary artery stenosis-safety & effectiveness of drug-eluting stents & anti-platelet regimen). JACC Cardiovasc Interv. 2013; 6:932–942. PMID:
24050860.
23. Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011; 305:1097–1105. PMID:
21406646.
24. Collet JP, Cuisset T, Rangé G, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012; 367:2100–2109. PMID:
23121439.
25. Cayla G, Cuisset T, Silvain J, et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet. 2016; 388:2015–2022. PMID:
27581531.
26. Roberts JD, Wells GA, Le May MR, et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet. 2012; 379:1705–1711. PMID:
22464343.
27. Parodi G, Marcucci R, Valenti R, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011; 306:1215–1223. PMID:
21934054.
28. Brar SS, ten Berg J, Marcucci R, et al. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data. J Am Coll Cardiol. 2011; 58:1945–1954. PMID:
22032704.
29. Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007; 50:309–315. PMID:
17659197.
30. Mak KH, Bhatt DL, Shao M, et al. Ethnic variation in adverse cardiovascular outcomes and bleeding complications in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study. Am Heart J. 2009; 157:658–665. PMID:
19332192.
31. Kang J, Palmerini KP, Stone G, et al. Ethnic differences in ischemia/bleeding risk tradeoff during antiplatelet therapy after drug-eluting stent implantation: individual patient level meta-analysis from seven RCTs. J Am Coll Cardiol. 2018; 71:A1320.
32. Kang J, Han JK, Ahn Y, et al. Third-generation P2Y12 inhibitors in East Asian acute myocardial infarction patients: a nationwide prospective multicentre study. Thromb Haemost. 2018; 118:591–600. PMID:
29534250.
33. Faber DR, de Groot PG, Visseren FL. Role of adipose tissue in haemostasis, coagulation and fibrinolysis. Obes Rev. 2009; 10:554–563. PMID:
19460118.
34. Levine GN, Jeong YH, Goto S, et al. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol. 2014; 11:597–606. PMID:
25154978.
35. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007; 357:2001–2015. PMID:
17982182.
36. Montalescot G, Wiviott SD, Braunwald E, et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009; 373:723–731. PMID:
19249633.
37. Smith PK, Goodnough LT, Levy JH, et al. Mortality benefit with prasugrel in the TRITON-TIMI 38 coronary artery bypass grafting cohort: risk-adjusted retrospective data analysis. J Am Coll Cardiol. 2012; 60:388–396. PMID:
22633653.
38. Olier I, Sirker A, Hildick-Smith DJ, et al. Association of different antiplatelet therapies with mortality after primary percutaneous coronary intervention. Heart. 2018; heartjnl-2017-312366.
39. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009; 361:1045–1057. PMID:
19717846.
40. Steg PG, James S, Harrington RA, et al. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010; 122:2131–2141. PMID:
21060072.
41. James S, Budaj A, Aylward P, et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2010; 122:1056–1067. PMID:
20805430.
42. James S, Angiolillo DJ, Cornel JH, et al. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2010; 31:3006–3016. PMID:
20802246.
43. Sahlén A, Varenhorst C, Lagerqvist B, et al. Outcomes in patients treated with ticagrelor or clopidogrel after acute myocardial infarction: experiences from SWEDEHEART registry. Eur Heart J. 2016; 37:3335–3342. PMID:
27436867.
44. Kang HJ, Clare RM, Gao R, et al. Ticagrelor versus clopidogrel in Asian patients with acute coronary syndrome: a retrospective analysis from the Platelet Inhibition and Patient Outcomes (PLATO) Trial. Am Heart J. 2015; 169:899–905.e1. PMID:
26027629.
45. Goto S, Huang CH, Park SJ, Emanuelsson H, Kimura T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome -- randomized, double-blind, phase III PHILO study. Circ J. 2015; 79:2452–2460. PMID:
26376600.
46. Saito S, Isshiki T, Kimura T, et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS study. Circ J. 2014; 78:1684–1692. PMID:
24759796.
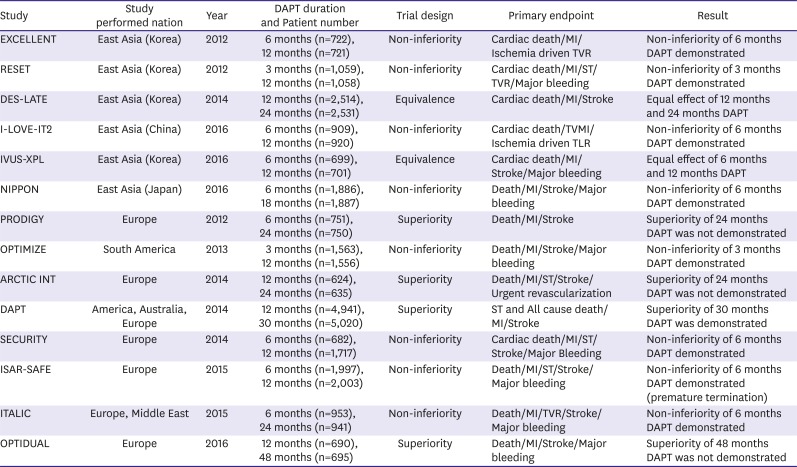
47. Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation. 2014; 129:304–312. PMID:
24097439.
48. Valgimigli M, Campo G, Monti M, et al. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012; 125:2015–2026. PMID:
22438530.
49. Collet JP, Silvain J, Barthélémy O, et al. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet. 2014; 384:1577–1585. PMID:
25037988.
50. Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014; 371:2155–2166. PMID:
25399658.
51. Gilard M, Barragan P, Noryani AA, et al. 6- versus 24-month dual antiplatelet therapy after implantation of drug-eluting stents in patients nonresistant to aspirin: the randomized, multicenter ITALIC trial. J Am Coll Cardiol. 2015; 65:777–786. PMID:
25461690.
52. Helft G, Steg PG, Le Feuvre C, et al. Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: the OPTIDUAL randomized trial. Eur Heart J. 2016; 37:365–374. PMID:
26364288.
53. Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012; 125:505–513. PMID:
22179532.
54. Kim BK, Hong MK, Shin DH, et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol. 2012; 60:1340–1348. PMID:
22999717.
55. Han Y, Xu B, Xu K, et al. Six versus 12 months of dual antiplatelet therapy after implantation of biodegradable polymer sirolimus-eluting stent: randomized substudy of the I-LOVE-IT 2 trial. Circ Cardiovasc Interv. 2016; 9:e003145. PMID:
26858080.
56. Hong SJ, Shin DH, Kim JS, et al. 6-month versus 12-month dual-antiplatelet therapy following long everolimus-eluting stent implantation: The IVUS-XPL randomized clinical trial. JACC Cardiovasc Interv. 2016; 9:1438–1446. PMID:
27212028.
57. Nakamura M, Iijima R, Ako J, et al. Dual antiplatelet therapy for 6 versus 18 months after biodegradable polymer drug-eluting stent implantation. JACC Cardiovasc Interv. 2017; 10:1189–1198. PMID:
28641838.
58. Feres F, Costa RA, Abizaid A, et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013; 310:2510–2522. PMID:
24177257.
59. Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol. 2014; 64:2086–2097. PMID:
25236346.
60. Schulz-Schüpke S, Byrne RA, Ten Berg JM, et al. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J. 2015; 36:1252–1263. PMID:
25616646.
61. Bittl JA, Baber U, Bradley SM, Wijeysundera DN. Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2016; 68:1116–1139. PMID:
27036919.
62. Patil RK, Swaminathan RV, Feldman DN. Continuation of dual-antiplatelet therapy following percutaneous revascularization with a drug-eluting stent: what duration is optimal? Curr Atheroscler Rep. 2015; 17:63. PMID:
26399876.
63. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018; 39:213–260. PMID:
28886622.
64. Bittl JA, Baber U, Bradley SM, Wijeysundera DN; Evidence Review Committee Members. Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2016; 134:e156–e178. PMID:
27026019.
65. Urban P, Meredith IT, Abizaid A, et al. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015; 373:2038–2047. PMID:
26466021.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download