This article has been
cited by other articles in ScienceCentral.
Abstract
The purpose of this study was to ascertain change in structure of mucosa of small intestine, if any, in small intestine of Swiss albino mice as an effect of chronic use of nonsteroidal anti-inflammatory drugs–Ibuprofen. Longitudinal study conducted on 46 adult Swiss albino mice, 23 as experimental and 23 as control. Ibuprofen was given at a dose of 40 µg/g body weight per day for 6 weeks by intragastric route in experimental group of mice while control group of mice received same volume of distilled water. Mice of both the groups were sacrificed and desired segments of small intestines were dissected out and tissues were subjected to histological processing. Histomorphometry was performed and relevant photomicrographs were obtained. Student's unpaired t test by GraphPad Prism 6 software. Height of villi was not significantly altered but there was significant reduction of the number of goblet and non-goblet cells (enterocytes and other columnar cells) in mucosal lining of the small intestine of experimental group of mice. Percent distribution of the goblet and non-goblet cells was not altered in villi of two groups. Chronic exposure of Ibuprofen in therapeutic dosage caused reduction of the functional cell mass in lining epithelium of villi of middle segment of small intestine. However, there was no evidence of ulcerative or hemorrhagic lesion.
Go to :

Keywords: Enterocytes, Goblet cells, Ibuprofen, Morphometry, Small intestine
Introduction
Formulation containing nonsteroidal anti-inflammatory drugs (NSAIDs) has become a common feature of almost every civilized household nowadays. Use of NSAID tablets, lotions, gel, spray, creams or injections is so rampant in society that even children are aware of many of their brand names. One might find a senior family member giving caution to others not to take such medications empty stomach since the untoward gastric side effects of NSAIDs have been well acknowledged by their users.
Unfriendly relationship of NSAID with stomach raises one doubt: “if stomach, why not intestine?” Whatever might be the cause of susceptibility of stomach to fall prey to NSAIDs, has small intestine got some natural defense against harmful effects of NSAID or it does fall prey to a lesser extent which may remain unnoticed clinically? Further, if NSAIDs do affect small intestine, what structural alterations do they produce? Some of these queries prompted us to perform this study. Latest literature review suggests an increasing awareness of clinicians towards causation of enteric side effects by selective as well as non selective NSAIDs [
1]. Protein losing enteropathy and occult blood in stools is the trivial form of it while intestinal erosions, ulcerations and haemorrhage have been reported to be the result of unmonitored prolonged NSAID therapy [
2]. Ibuprofen is a propionic acid derivative belonging to non selective NSAID group. In many chronic inflammatory conditions such as rheumatoid arthritis, osteo arthrosis etc it has been prescribed as single or combined medication by clinicians and considered safe for prolonged medication.
Present study was a case-control based histological study which was done on two groups of adult Swiss albino mice. One group received NSAID-ibuprofen while other acted as a control. Microscopic examination and histo-morphometry of intestinal sections of two groups were done and relevant photomicrographs were obtained. These two groups were compared. The principal aim was to demonstrate structural change, if any, in mucosa of small intestine of adult Swiss albino mice as an effect of chronic use of NSAID-ibuprofen.
Go to :

Materials and Methods
This was the case control study done on 46 adult Swiss albino mice of either sex and they were divided in two equal groups, 23 as experimental and 23 as control. Standard protocol of animal experimentation [
3] was followed and permission from Institutional Animal Ethics Committee was obtained. Sexes were kept separate to prevent mating. After a weeklong drug free period for acclimatisation of newly procured animals, experimental group of animals were given aqueous ibuprofen suspension at a dose of 40 µg/g orally by gastric gavage using graduated tuberculin syringe fitted with feeding canula for a period of 6 weeks. Control group of mice received appropriate volume of distilled water as vehicle base. Mice of both groups were sacrificed by cervical dislocation; desired segments of intestine (jejunum) were dissected out and subjected to routine histological processing [
4]. A randomly selected fresh segment of small intestine was cut and opened out from ante-mesenteric border to examine its interior using a magnifying glass (
Fig. 1).
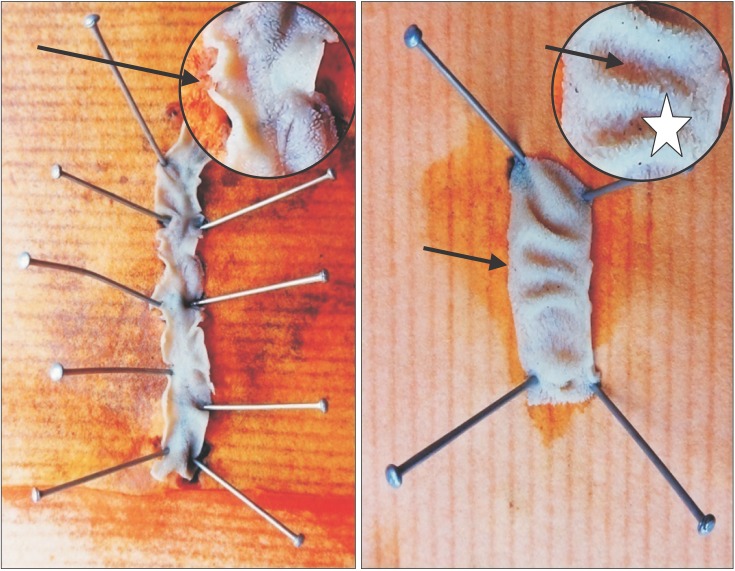 | Fig. 1Gross examination of the interior of intestinal segment: note normal appearance of plicae circulares (*) and ‘villi’ as furry coat (arrows).
|
Stained sections of intestine (with hematoxylin and eosin [H&E]; Alcian blue–hematoxylin) were examined under microscope, histomorphometry of desired parameter was performed, data entered in tables and selective photomicrographs were obtained as record.
Method of histomorphometric study
Height of villi
It was measured under microscope using pre calibrated ocular micrometer scale. A given microscopic field was considered or rejected for morphometry based on following criteria:
- Inclusion criteria: Villi representative but free from artifacts and technical faults were selected. Threfore, villi were chosen amongst the attached and longitudinally cut villi showing stromal core in continuation with the intercryptal stroma and its epithelial covering showing single row of nuclei continuous with the intervillous epithelium on either side [
5].
- Exclusion criteria: Unattached villi were assumed to represent sections through the tops of obliquely oriented or inclined villi and they were excluded. Grossly deformed villi, villi with heaped up epithelial lining and disrupted villi were also excluded from morphometry.
Height of 25 villi was measured in each animal and a mean value for the animal was obtained. Based on mean values of individual animals of a group, a mean value of height of villus was obtained for the whole group. The difference in the heights of two groups of villi was then obtained with its statistical significance (
Table 1).
Table 1
Comparison of two groups
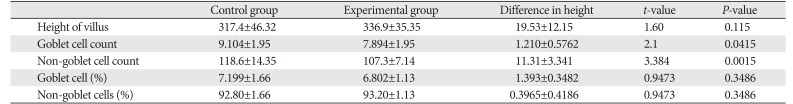
|
Control group |
Experimental group |
Difference in height |
t-value |
P-value |
|
Height of villus |
317.4±46.32 |
336.9±35.35 |
19.53±12.15 |
1.60 |
0.115 |
|
Goblet cell count |
9.104±1.95 |
7.894±1.95 |
1.210±0.5762 |
2.1 |
0.0415 |
|
Non-goblet cell count |
118.6±14.35 |
107.3±7.14 |
11.31±3.341 |
3.384 |
0.0015 |
|
Goblet cell (%) |
7.199±1.66 |
6.802±1.13 |
1.393±0.3482 |
0.9473 |
0.3486 |
|
Non-goblet cells (%) |
92.80±1.66 |
93.20±1.13 |
0.3965±0.4186 |
0.9473 |
0.3486 |

Method of counting epithelial cells in villus lining
The epithelial cells lining the mucosa were examined from the villus tip to the bottom of crypt. Enterocytes and goblet cells could be clearly identified with conventional H&E staining. Alcian blue stained sections were specifically used for counting goblet cells. The columnar cells other than goblet cells in the lining were considered together as non goblet cells (largely representing enterocytes). For counting goblet and ‘non-goblet’ cells on villus lining, ten most suitable villi (as per inclusion criteria) were chosen from different sections of intestine of a single animal. The mean value for both, goblet and non goblet cells, was obtained for each animal and then a mean value for the group was obtained. The difference in the mean number of two groups was calculated and its statistical significance was obtained (
Table 1).
From the values of mean numbers of goblet and non goblet cells per villus, the percent distribution of these cells per villus was obtained (
Table 1,
Figs. 2,
3).
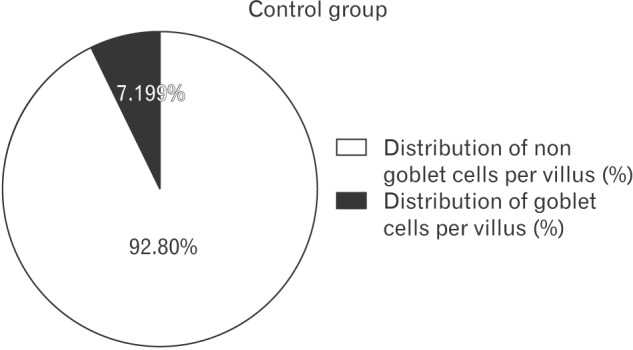 | Fig. 2Distribution of goblet and non-goblet cells per villus (%) in control group shown by pie diagram.
|
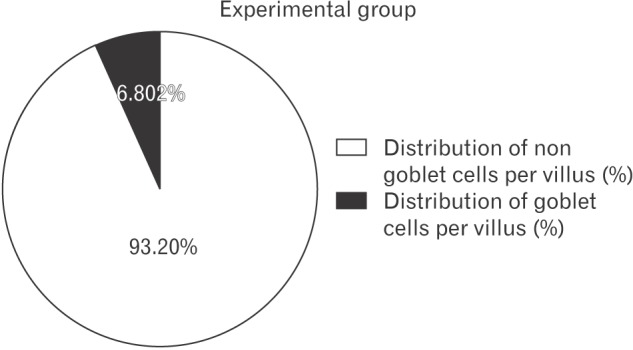 | Fig. 3Distribution of goblet and non-goblet cells per villus (%) in experimental group shown by pie diagram.
|
Statistical method
Data was expressed in mean±standard deviation and the analysis of the data was done using GraphPad Prism 6 software (GraphPad Software Inc., San Diego, CA, USA). Statistical difference between the data obtained in two groups was evaluated by Student's unpaired t test and P-value of <0.05 was considered as statistically significant.
Go to :

Results
Gross examination of intestinal segments of two groups did not reveal any noticeable pathology externally or internally. Since a detailed histological account of Swiss albino mice intestine was not traceable in literature, we compared the findings of control group with experimental, considering the findings of control group to be representative of general mice population and any deviation in experimental group to be result of ibuprofen exposure. Qualitatively there was not much alteration in microscopic structure of the mucosa of the small intestine. However, there was more degree of distortion of villi and inflammatory cell infiltration in experimental group of mice. No animal had ulcerative or haemorrhagic lesion. In general, the villi appeared scarce in distribution in experimental group but the picture was not consistent in all animals. The number of goblet cells was comparatively less on villi as compared to crypts in both groups. There was no evidence of increased mitotic activity in crypts and the number and sizes of the crypt in two groups of animals was more or less same.
Morphometry of the height of villi in two groups, count of goblet cells and non goblet cells per villi and percent distribution of goblet and non goblet cells per villi was tabulated as follows (
Table 1).
Section of small intestine showing distribution of goblet cell, goblet cell, enterocyte, and villous core in control and experimental group is shown in
Figs. 4,
5,
6,
7.
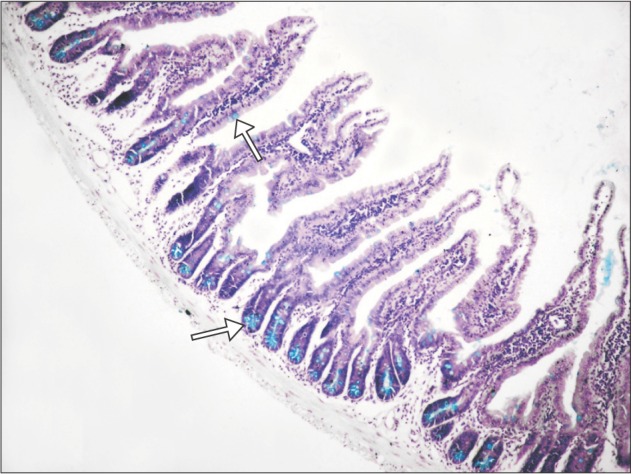 | Fig. 4Section of small intestine showing distribution (arrows) of goblet cells (control group; Alcian blue, ×100).
|
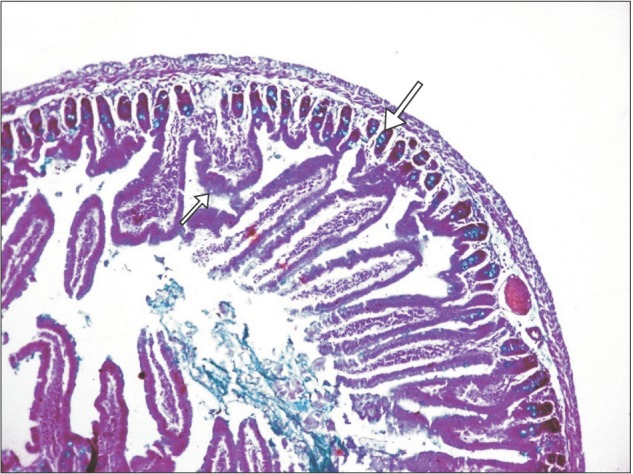 | Fig. 5Section of small intestine showing distribution (arrows) of goblet cells (experimental group; Alcian blue, ×100).
|
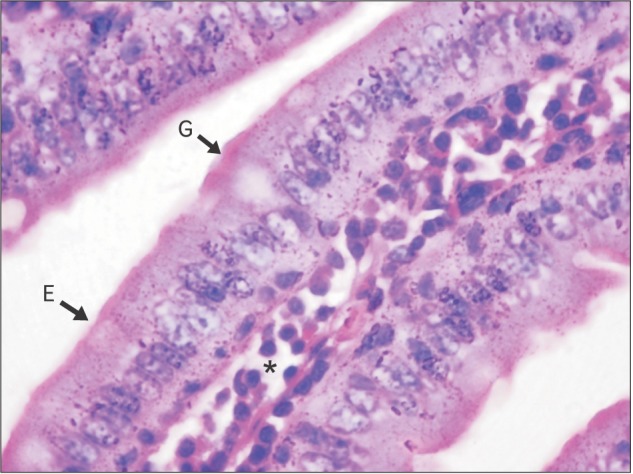 | Fig. 6Section of small intestine showing goblet cell (G), enterocytes (E), and villus core (*) (control; H&E, 1,000).
|
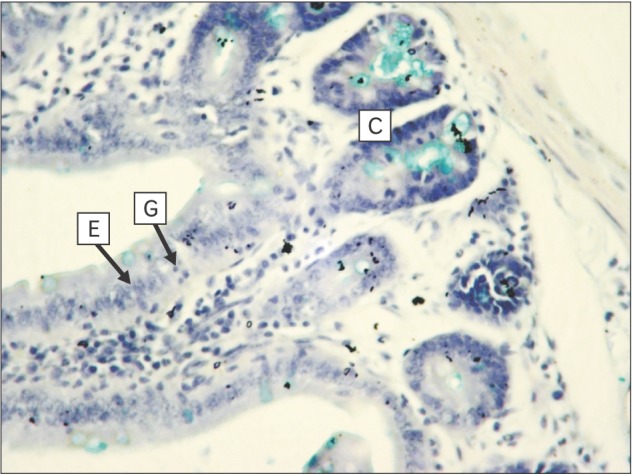 | Fig. 7Section of small intestine showing goblet cells (G), enterocytes (E) on villus with villus core (*) and crypt (C) (experimental; Alcian blue, ×400).
|
Go to :

Discussion
In spite of vigorous search in literature we did not find any study exactly matching with present study to compare. Studies so far performed on the related topic confine to the comparison of clinical or biochemical data as an effect of NSAIDs in either human volunteer or other mammals. However similar studies performed on mice or rats with similar groups of drugs are scarcely available and so histological finding of such studies have been taken into account.
We noted an apparent increase in heights of villi in experimental group though it was not statistically significant. It was inferred as a mixed parameter of increased or decreased heights of different villi of animals of the same group. In absence of any consistent finding we considered villus height to be largely unchanged. Anthony et al. [
6] and Kuroda et al. [
7] have reported drastic reduction of villus height and number after giving acute dosage of NSAIDs.
In present study the number of goblet cells and other columnar cells (non-goblet cells) were found to be significantly reduced in number on epithelial lining of villi. Ettarh and Carr [
5] also noted reduction in number of columnar cells on villi as an effect of two intraperitoneal injections of indomethacin in mice. This finding matches with present study. Ettarh and Carr [
5] have also noticed increase in number of crypts, more mitotic activity in walls of crypt, increase in number of columnar cells in crypts and increase in number of goblet cells both in villi as well as crypts. They correlated such proliferative activities to be the result of ulcerogenic effect of indomethacin in small intestine of mouse.
Though we have not seen damaged cells or degenerative cells in epithelium but end result in the form of reduction in number of cells is indicative of this feature. However, in present study the percent distribution of goblet and non goblet columnar cells in villi was largely unchanged in two groups of mice. Reduction in number of cells in spite of the normal proportion of the cells and unaffected height of villus is indicative of a net loss of cells in villi of experimental group. Though the loss of cells was trivial and almost unnoticeable in present study, it bears importance in view of a direct cellular toxicity of NSAIDs for being irritant acids in nature [
8].
Dixon and Paulley [
9] are of the opinion that mucin produced by goblet cells offers a protective coat to the columnar absorptive cells of mucosa especially when motility of wall is reduced as an effect of antispasmodic drugs such as mecamylamine. They demonstrated an increase in size and number of goblet cell in response to exposure of this drug in rat intestine. Reduction in number of goblet cells would obviously deprive epithelial cells of their mucin coat and this would render them more prone to be affected by direct irritant effect of NSAIDs as well as ingress of bacteria from lumen to wall. A residual form of mucosal lesion was suggested by the infiltration of leucocytes. However, this feature was inconsistent in two groups. Free radical damage due to accumulation of neutrophils in mucosa has been postulated by many workers to be the cause of NSAID induced mucosal damage in intestine, with which we partially agree [
810].
In absence of histomorphometric literature to compare with our findings, we are of the opinion that ibuprofen in prolonged therapeutic doses is potentially toxic to the small intestinal mucosa of Swiss albino mice, though the effect might get compensated by the body's defense and may not reveal by a gross change in appearance or consistency of faeces. Morphometric findings of the present study suggest that ibuprofen might have caused depletion of functional cell mass on the villi of small intestinal mucosa.
This experimentation need to be carried out on a larger scale to reach at a concrete conclusion about safety of ibuprofen in small intestine on prolonged medication.
Go to :

Acknowledgements
Authors gratefully acknowledge the technical support provided by Mr. V. P. Kavinesan in photomicrophs.
Go to :

References
1. Tacheci I, Kopacova M, Rejchrt S, Bures J. Non-steroidal anti-inflammatory drug induced injury to the small intestine. Acta Medica (Hradec Kralove). 2010; 53:3–11. PMID:
20608226.
2. Smale S, Tibble J, Sigthorsson G, Bjarnason I. Epidemiology and differential diagnosis of NSAID-induced injury to the mucosa of the small intestine. Best Pract Res Clin Gastroenterol. 2001; 15:723–738. PMID:
11566037.
3. Chatterjee TK. Handbook of laboratory mice and rats. Calcutta: K.K. Chatterjee Publication;1993. p. 3–12.
4. Drury RA, Wallington EA. Carleton's histological technique. 5th ed. New York: Oxford University Press;1980. p. 57–75.
5. Ettarh RR, Carr KE. Morphometric analysis of the small intestinal epithelium in the indomethacin-treated mouse. J Anat. 1996; 189(Pt 1):51–56. PMID:
8771395.
6. Anthony A, Dhillon AP, Nygard G, Hudson M, Piasecki C, Strong P, Trevethick MA, Clayton NM, Jordan CC, Pounder RE, Wakefield AJ. Early histological features of small intestinal injury induced by indomethacin. Aliment Pharmacol Ther. 1993; 7:29–39. PMID:
8439635.
7. Kuroda M, Yoshida N, Ichikawa H, Takagi T, Okuda T, Naito Y, Okanoue T, Yoshikawa T. Lansoprazole, a proton pump inhibitor, reduces the severity of indomethacin-induced rat enteritis. Int J Mol Med. 2006; 17:89–93. PMID:
16328016.
8. Menozzi A, Pozzoli C, Giovannini E, Solenghi E, Grandi D, Bonardi S, Bertini S, Vasina V, Coruzzi G. Intestinal effects of non-selective and selective cyclooxygenase inhibitors in the rat. Eur J Pharmacol. 2006; 552:143–150. PMID:
17069793.
9. Dixon JM, Paulley JW. Bacteriological and histological studies of the small intestine of rats treated with mecamylamine. Gut. 1963; 4:169–173. PMID:
14028129.
10. Fortun PJ, Hawkey CJ. Nonsteroidal antiinflammatory drugs and the small intestine. Curr Opin Gastroenterol. 2007; 23:134–141. PMID:
17268241.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download