Dear Editor:
Stevens-Johnson syndrome (SJS) is a life-threatening skin reaction characterized by extensive epidermal detachment1. Drugs, especially sulfa drugs, anti-epileptics, and antibiotics, are the most common causes1, but recently, SJS associated with herbal medication has been reported2. Herein, we report a case of SJS probably induced by herbal medicine. We received the patient's consent form about publishing all photographic materials. The study protocol was approved by the Institutional Review Board of Incheon St. Mary's Hospital, The Catholic University of Korea (IRB no. OC17ZESI0049).
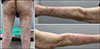
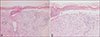
A 77-year-old man presented with a sudden onset of bullous lesions on his trunk and extremities (Fig. 1). The histopathological findings were sub-epidermal split with extensive epidermal necrosis (Fig. 2). The direct immunofluorescence findings were negative. Days after the skin biopsy, the vesicles and bullae began to fuse, rapidly progressing into skin erosion and denudation. The mucous membranes of the mouth and conjunctiva were also affected. Epidermal detachment was seen in less than 10% of the body surface area and the Nikolsky sign was present. The patient answered that there has been no change in his routine medication for the past 3 years, but mentioned that he started on herbal medication a month ago. The herbal medication was said to contain deer antlers, ginseng, camphor etc. Based on severity-of-illness score for toxic epidermal necrolysis, our patient's expected mortality rate was 12.1%. He was asked to immediately stop the herbal medication. The patient made a full recovery after a course of intravenous steroid therapy, daily dressings and supportive care.
According to prior reports, the patch test results varied among SJS patients with culprit drug. Lin et al.3 reported that while 62.5% of patients with carbamazepine-induced SJS/toxic epidermal necrolysis (TEN) show positive patch test results, patch tests for allopurinol-induced SJS/TEN are mostly negative. As for our case, we were not able to perform a patch test on herbal medicine because the patient refused.
Differential diagnoses of SJS include exfoliative dermatitis, staphylococcal scalded skin syndrome (4S), bullous pemphigoid (BP), paraneoplastic pemphigus and bullous erythema multiforme (EM). Although exfoliative dermatitis resembles SJS clinically, it rarely affects the mucosa. There is an intra-epidermal separation in 4S unlike SJS, which shows a sub-epidermal split. BP usually shows a gradual onset and spares the mucosal area. Paraneoplastic pemphigus is usually associated with an underlying cancer. As for our patient, the cancer screening tests were negative. Bullous EM is commonly triggered by herpes simplex virus infection and presents with characteristic targetoid lesions.
More than 100 drugs have been reported as potential cause of SJS4, but herbal medicine induced SJS has not yet been reported in the Korean literature. Recently, several studies have reported the relationship between the human leukocyte antigen (HLA) genotype and drug hypersensitivity. Although the HLA genotype of the herbal induced SJS remained uncertain. Herbal medications carry a mixture of ingredients that originate from plants and animals. Because of this characteristic, identifying the culprit ingredient within the herbal medication is extremely difficult. Also, since there is no obligation to notify the ingredient within the medication packet, scientific evaluation in case of adverse events are nearly impossible. Herbal medication induced drug eruption can be caused by herbal medicine itself, but also by added impurities and the combination of ingredients5. With the lack of patch testing and being unable to identify the full ingredient of the herbal medicine, the authors were not able to confirm that our SJS is caused by herbal medicine. However with the clinical circumstances, we believe that herbal medicine is most likely the culprit drug in our case. We report this case because we think it is important that dermatologists consider the possibility of herbal medicine- induced drug eruption and notify the public about the potential serious side effects of herbal medicine.
Figures and Tables
References
1. Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens-Johnson syndrome: a review. Crit Care Med. 2011; 39:1521–1532.

2. Bonhomme A, Poreaux C, Jouen F, Schmutz JL, Gillet P, Barbaud A. Bullous drug eruption to Nigella sativa oil: Consideration of the use of a herbal medicine - clinical report and review of the literature. J Eur Acad Dermatol Venereol. 2017; 31:e217–e219.
3. Lin YT, Chang YC, Hui RC, Yang CH, Ho HC, Hung SI, et al. A patch testing and cross-sensitivity study of carbamazepine-induced severe cutaneous adverse drug reactions. J Eur Acad Dermatol Venereol. 2013; 27:356–364.

4. Yoon J, Oh CW, Kim CY. Stevens-johnson syndrome induced by vandetanib. Ann Dermatol. 2011; 23:Suppl 3. S343–S345.

5. Lim YL, Thirumoorthy T. Serious cutaneous adverse reactions to traditional Chinese medicines. Singapore Med J. 2005; 46:714–717.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download