Abstract
Drug induced lichen planus like eruption is an uncommon cutaneous adverse effect of several drugs. This appears symmetric eruption of erythematous or violaceous plaques resembling lichen planus on the trunk and extremities. A 50-year-old male presented with scaly, violaceous plaques and dusky brown macules on whole body. For four months, the patient was treated with olmutinib, an oral, third-generation epidermal growth factor receptor-tyrosine kinase inhibitor. In May 2016, olmutinib received its first global approval in South Korea for the treatment of patients with locally advanced or metastatic epidermal growth factor receptor T790M mutation-positive non-small cell lung cancer. The biopsy specimen from the patient showed features of lichen planus. We diagnosed him with olmutinib-induced lichen planus like eruption. He was treated with oral methylprednisolone and topical desoxymethasone 0.25% ointment. At the same time, olmutinib dose was decreased to three-fourths of this patient's starting dose. After that, the cutaneous lesions improved.
Olmutinib is an oral, third-generation epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) for locally advanced or metastatic EGFR T790M mutation-positive non-small cell lung cancer. This received its first global approval in South Korea in May 20161. We present a case of lichen planus (LP) like eruption in a man after olmutinib treatment. To our knowledge, this is the first reported case of olmutinib induced LP like eruption.
A 50-year-old male presented with scaly, violaceous plaques and dusky brown macules on whole body for one month (Fig. 1A). Flexural areas were spared (Fig. 1B). There was no mucous membrane lesion. The patient had been treated with olmutinib for four months because of non-small cell lung cancer. Pleural and brain metastasis were found at the time of diagnosis. Therefore, clinical trial with olmutinib for palliative chemotherapy was performed with patient consent. No other medication was used to this patient within three months. Skin biopsy specimen of his dorsum of hand demonstrated focal orthokeratosis, wedge-shaped hypergranulosis, saw-toothed irregular elongated rete ridges, and dermal lichenoid lymphocytic infiltration with scanty eosinophils. Max-Joseph space and dyskeratotic cells were also shown in the specimen (Fig. 2). These histologic features resembled those of LP. However, the clinical features were different from typical features of idiopathic LP, and the cutaneous lesions appeared after olmutinib therapy. Consequently, we diagnosed this olmutinib induced LP like eruption. He was treated with oral methylprednisolone 20 mg per day initially. Every three days, the dosage of methylprednisolone was reduced in half than before. Topical desoxymethasone 0.25% ointment was also applied to his lesions. At the same time, olmutinib dosage was decreased to three-fourths of this patient's starting dose (800 mg per day to 600 mg per day), because the oncologist considered this cutaneous reaction as grade III adverse event. After three weeks, the body surface area of cutaneous lesions decreased to about a half. After the discontinuation of systemic corticosteroid therapy, he was treated with oral antihistamine, topical steroid and tacrolimus ointments for several weeks because of itch. Although the dosage of olmutinib has been maintained 600 mg per day for four months, new lesions have not appeared.
Drug induced LP like eruption is an uncommon cutaneous adverse event of several drugs such as antimalarial, beta-blockers, gold salts, methyldopa, or quinidine2. Latency period varies from months to a year or more. It depends upon the class of drug, dose, host reaction, and concurrent medications3. Typical cutaneous lesion of drug induced LP like eruption is symmetric eruption of erythematous or violaceous plaques on the trunk and extremities resembling idiopathic LP. However, drug induced LP like eruption rarely shows the flexural distribution which is common in idiopathic LP. Furthermore, mucous membrane involvement is less common in drug induced LP like eruption4.
In this case, skin lesions developed after the initiation of olmutinib therapy and improved after decreasing of dosage of olmutinib. Flexural areas were spared. Furthermore, mucous membranes were not involved. The histopathologic features show those of idiopathic LP. Therefore, we called this case as ‘olmutinib induced LP like eruption.’
There are several reports of cutaneous adverse events associated with biologics or targeted therapy. For example, tumor necrosis factor-α inhibitors are well known as the one of agents causing LP like eruption. EGFR-TKIs can have major skin toxic effects including acneiform eruption, pruritus, xerosis, and paronychia56. However, we cannot find the literatures about LP, LP like eruption, or lichenoid drug eruption associated with the first- and second-generation EGFR-TKIs, gefitinib, erlotinib, and afatinib. There is no reported case of LP like eruption related to EGFR inhibitors in PubMed. On the other hand, other TKIs except EGFR-TKIs can cause LP or LP like eruption, with 2 literatures, 9 cases reported to date in PubMed78. Some authors hypothesized that signal inhibition of platelet-derived growth factor receptor, c-kit receptor or Src family kinase by TKIs may lead to keratinocytic apoptosis and lymphocytic inflammation7. However, TKIs used in their literature are first-, second-, or third-generation TKIs such as imatinib, nilotinib, dasatinib, nilotinib, or ponatinib78.
There is no reported literature of cutaneous adverse events of olmutinib. Pathomechanism of olmutinib induced LP like eruption is also uncertain. Tyrosine kinases can inhibit not only mutant EGFR, but also wild-type EGFR9. Olmutinib is an irreversible kinase inhibitor, binding to a cysteine residue near the kinase domain, and known as having little effect on cell lines with wild-type EGFR10. Theoretically, olmutinib should have minimal effect to normal skin, but LP like eruption was occurred in our case. Serious cutaneous adverse events of olmutinib, toxic epidermal necrolysis and Stevens-Johnson syndrome, also became an issue a few months ago. Therefore, we claim that clinicians should be aware of the risk of cutaneous adverse events of olmutinib.
Figures and Tables
Fig. 1
(A) Scaly, violaceous to black-colored plaques and dusky brown macules on trunk. (B) Same lesions are at both thighs and calves. However, popliteal areas were spared.
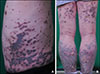
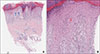
Fig. 2
Histopathological images of skin biopsy. (A) Orthokeratosis, wedge-shaped hypergranulosis, saw-toothed irregular elongated rete ridges in the epidermis. Max-Joseph spaces are shown (blue arrows). Lichenoid lymphocytic infiltration in the dermis (H&E, ×40). (B) Dyskeratotic cells in the epidermis (red arrow) (H&E, ×200).
References
3. Brauer J, Votava HJ, Meehan S, Soter NA. Lichenoid drug eruption. Dermatol Online J. 2009; 15:13.

4. Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, et al. Fitzpatrick's dermatology in general medicine. 8th ed. New York: McGraw-Hill Medical;2012. p. 304.
5. Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006; 55:657–670.

6. Lacouture ME, Schadendorf D, Chu CY, Uttenreuther-Fischer M, Stammberger U, O'Brien D, et al. Dermatologic adverse events associated with afatinib: an oral ErbB family blocker. Expert Rev Anticancer Ther. 2013; 13:721–728.

7. Patel AB, Solomon AR, Mauro MJ, Ehst BD. Unique cutaneous reaction to second- and third-generation tyrosine kinase inhibitors for chronic myeloid leukemia. Dermatology. 2016; 232:122–125.

8. Pretel-Irazabal M, Tuneu-Valls A, Ormaechea-Perez N. [Adverse skin effects of imatinib, a tyrosine kinase inhibitor]. Actas Dermosifiliogr. 2014; 105:655–662. Spanish.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download