This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
This study aimed to evaluate the surgical outcomes and investigate the feasibility of reduced-port laparoscopic gastrectomy using learning curve analysis in a small-volume center.
Materials and Methods
We reviewed 269 patients who underwent laparoscopic distal gastrectomy (LDG) for gastric carcinoma between 2012 and 2017. Among them, 159 patients underwent reduced-port laparoscopic gastrectomy. The cumulative sum technique was used for quantitative assessment of the learning curve.
Results
There were no statistically significant differences in the baseline characteristics of patients who underwent conventional and reduced-port LDG, and the operative time did not significantly differ between the groups. However, the amount of intraoperative bleeding was significantly lower in the reduced-port laparoscopic gastrectomy group (56.3 vs. 48.2 mL; P<0.001). There were no significant differences between the groups in terms of the first flatus time or length of hospital stay. Neither the incidence nor the severity of the complications significantly differed between the groups. The slope of the cumulative sum curve indicates the trend of learning performance. After 33 operations, the slope gently stabilized, which was regarded as the breakpoint of the learning curve.
Conclusions
The surgical outcomes of reduced-port laparoscopic gastrectomy were comparable to those of conventional laparoscopic gastrectomy, suggesting that transition from conventional to reduced-port laparoscopic gastrectomy is feasible and safe, with a relatively short learning curve, in a small-volume center.
Keywords: Stomach neoplasms, Gastrectomy, Laparoscopy, Learning curve
INTRODUCTION
Since the introduction of laparoscopic-assisted distal gastrectomy in 1994 [
1], it has been widely used to treat early gastric cancer in Asian countries [
2]. Several studies have reported that the procedure is associated with less pain, lower morbidity, and a shorter hospital stay than open surgery [
3]. Further, many surgeons have tried to further reduce the invasiveness of laparoscopic surgery by reducing the number and size of abdominal ports; this approach is known as “reduced-port laparoscopic surgery” [
4].
Recently, clinicians have begun to implement single-port or reduced-port laparoscopic surgery to treat gastric cancer [
567891011]. However, the technique typically requires specialized instruments, such as multichannel ports, a flexible scope, and a specialized organ retractor to lift the stomach, which may limit its use [
56789]. Previously, these specialized instruments were considered essential to overcome technical difficulties such as restricted surgical view and limited instrument movement during reduced-port laparoscopic surgery. However, Kim et al. [
9] recently introduced a novel approach, namely “duet laparoscopic distal gastrectomy (duet-LDG),” which can be performed by an operator and a laparoscopist using only three abdominal ports. Duet-LDG requires fewer assistants than does conventional LDG, and it uses operative techniques and instruments similar to those used in conventional LDG.
Previous studies on the feasibility and safety of reduced-port laparoscopic gastrectomy have usually been carried out in large-volume centers with extensive experience of laparoscopic gastrectomy. In our institution, conventional 5-port gastrectomy has been performed in the past. However, reduced-port laparoscopic gastrectomy using 3 ports is now routinely performed without special instruments or an assistant. The present study aimed to evaluate the surgical outcomes of our techniques and to investigate the feasibility of reduced-port laparoscopic gastrectomy using learning curve analysis in a small-volume center.
MATERIALS AND METHODS
Patients
The present study was approved by the Institutional Review Board of our institution (Chonnam National University Hwasun Hospital, Hwasun, Korea). Between January 2012 and December 2017, 283 patients with gastric cancer underwent LDG. All procedures were performed by a single surgeon who had a previous experience of ≥60 gastrectomy cases/year. Before May 2014, 110 patients underwent conventional LDG using 5 abdominal ports (conventional laparoscopic gastrectomy; CPG group). Reduced-port LDG was adopted for every patient in May 2014, and 173 patients subsequently underwent this procedure. Of these, 14 were excluded because they underwent LDG using 4 abdominal ports shortly after reduced-port LDG was introduced. Finally, 159 patients who underwent reduced-port distal gastrectomy were enrolled in the present study (reduced-port gastrectomy; RPG group). We compared the groups with respect to short-term surgical outcomes, including operative results, hospital course, morbidity, and mortality.
Before surgery, all patients underwent gastroduodenoscopy with biopsy, abdominal computed tomography, chest radiography, and laboratory testing. The indication for laparoscopic gastrectomy was clinical stage cT1-2N0. The gastric resection and regional lymph node dissection procedures followed the Japanese gastric cancer treatment guidelines [
12]. D1+ lymph node dissection was generally performed, and D2 lymph node dissection was indicated in cases of cT2 tumors or suspected lymph node metastasis. After surgery, patients were managed using standardized care protocols, including early active ambulation, pain control with epidural anesthesia, prophylaxis to prevent deep vein thrombosis, oral nutrition (from postoperative day [POD] 2), and hospital discharge between POD 6 and POD 8, depending on when the patient fulfilled the discharge criteria.
Data collection and definition
The following clinicopathological data were prospectively collected: age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) physical status classification, abdominal surgery history, laboratory results, and pathological data (including tumor, node, metastasis [TNM] stage). Operative parameters were also collected, namely the type of operative procedure, operative time, intraoperative blood loss, and the total number of retrieved lymph nodes. The following postoperative outcomes were measured: time to first flatus, time to diet resumption, duration of hospital stay, and postoperative complications, which were classified into local or systemic complications. Postoperative morbidity and mortality were defined as any complications or death occurring within 30 days of the operation or during hospitalization. The severity of the complications was graded using the Clavien-Dindo classification [
13], and pathological staging was based on the seventh edition of the Union Internationale Contre le Cancer/American Joint Committee on Cancer TNM classification system [
14].
Surgical technique of reduced-port laparoscopic gastrectomy
The patients were placed in the reverse Trendelenburg position, with their legs apart. The operator stood on the right side of the patient, and the laparoscopist stood between the legs of the patient. An umbilical port was inserted for the laparoscope, and pneumoperitoneum was established. Two operator ports were then placed in the right subcostal area (5-mm port) and right mid-abdomen (12-mm port) (
Fig. 1A). The liver was retracted using a straight needle with nylon sutures. The nylon suture was passed percutaneously, the middle portion was placed in the gastrohepatic ligament with a hemolock, and both ends were fixed on the skin using mosquito forceps (
Fig. 1B). All procedures, including omentectomy, lymph node dissection, gastric resection, and reconstruction, were performed using the instruments used for CPG. No special devices were used for retraction or counter-traction of the stomach.
 | Fig. 1Operative images of reduced-port laparoscopic gastrectomy. (A) Placement of abdominal ports; (B) Liver retraction using nylon suture; (C) Suprapancreatic lymph node dissection; (D) Removal of lymph node No. 11p.
|
Lymph node dissection at the suprapancreatic area was usually performed without compressing the pancreas (
Fig. 1C). However, in some cases that required D2 lymph node dissection, an additional 5-mm assistant port was added to compress the upper border of the pancreas and to better secure the operating field. During suprapancreatic lymph node dissection, the operating field for the suprapancreatic area was secured by compressing the pancreas with the right hand. Starting from the dissection of the soft tissue at the upper border of the pancreas, lymph nodes were removed in the following order: No. 8a, 12a, 11p, 9a, and 7 (
Fig. 1D). During the dissection of lymph node No. 11p, a gauze was used to prevent the stomach from descending. In laparoscopy-assisted distal gastrectomy (LADG), resection of the stomach and extracorporeal Billroth-I or -II anastomosis was performed through a 5–6-cm epigastric incision. During RPG, intracorporeal Billroth-II or Roux-en-Y reconstruction was performed for most patients, and the umbilical port was extended to 2–3 cm to extract the specimen. In early RPG, we performed extracorporeal anastomosis with 5–6-cm mini-laparotomy in some patients, which was similar to that performed in conventional LDG. After adoption of intracorporeal anastomosis, most patients in the later period of RPG underwent intracorporeal anastomosis.
Surgical technique of conventional port gastrectomy
In the CPG group, 5 abdominal ports were used: two bilateral subcostal ports, 2 bilateral mid-abdominal ports, and an umbilical port. The method of gastric resection and lymph node dissection was nearly identical to that of RPG.
Cumulative sum (CUSUM) analysis
The CUSUM technique was used for quantitative assessment of the learning curve. The CUSUM is the running total of differences between individual data points and the mean of all data points. The CUSUM of the operative time (CUSUMRPG) was defined as follows:
where
xi denotes the operative time for each patient and µ, the mean of the operative time in all patients. The slope of the CUSUM curve indicates the trend of learning performance, and the portion where the slope gently stabilizes is regarded as the breakpoint of the learning curve [
15]. In the present study, learning curves and CUSUM curves were obtained for the initial 86 patients. Subsequent operations were excluded, because resident doctors assisted in procedures such as liver retraction and reinforcement suture, which might have affected the operative time but not the surgical outcomes.
Statistical analysis
All data were analyzed using SPSS software version 23.0 (IBM Corp., Armonk, NY, USA). Continuous data were expressed as mean±standard deviation and compared using the Student's t-test. Categorical variables were expressed as number (%) and compared using the chi-square test or Fisher's exact test. Two-sided P-values less than 0.05 were considered statistically significant. A Microsoft Excel 2010 (Microsoft, Los Angeles, CA, USA) line chart was used for the CUSUM method.
RESULTS
Patient characteristics
There were 110 patients in the CPG group and 159, in the RPG group.
Table 1 shows the baseline characteristics of the patients in both the groups. The mean age of the patients (201 men and 68 women) was 63.9±10.6 years and their mean BMI was 23.6±3.2 kg/m
2, with 148 patients (55%) having underlying comorbidities. In total, 27 patients (10%) had undergone prior abdominal surgery. Patients in the RPG group did not show significant differences from those in the CPG group with respect to age, sex, BMI, ASA classification, or history of abdominal surgery. None of the following histopathological characteristics differed significantly between the groups: number of tumors, tumor location and size, differentiation, and pathological TNM (pTNM).
Table 1
Patient characteristics

|
Characteristics |
All (n=269) |
CPG (n=110) |
RPG (n=159) |
P |
|
Age (yr) |
63.9±10.6 |
62.8±10.2 |
64.7±10.7 |
0.135 |
|
Sex |
|
|
|
0.098 |
|
Male |
201 (74.7) |
88 (80) |
113 (71.1) |
|
Female |
68 (25.3) |
22 (20) |
46 (28.9) |
|
BMI (kg/m2) |
23.6±3.2 |
23.3±2.6 |
23.8±3.5 |
0.233 |
|
ASA classification |
|
|
|
0.532 |
|
I |
121 (45) |
53 (48.2) |
68 (42.8) |
|
II |
140 (52) |
53 (48.2) |
87 (54.7) |
|
III |
8 (3) |
4 (3.6) |
4 (2.5) |
|
Abdominal operation history |
|
|
|
0.102 |
|
No |
242 (90) |
95 (86.4) |
147 (92.5) |
|
Yes |
27 (10) |
15 (13.6) |
12 (7.5) |
|
No. of tumors |
|
|
|
0.640 |
|
1 |
254 (94.4) |
103 (93.6) |
151 (95) |
|
2 |
15 (5.6) |
7 (6.4) |
8 (5.0) |
|
Tumor location |
|
|
|
0.120 |
|
Lower 1/3rd
|
196 (72.9) |
82 (74.5) |
114 (71.7) |
|
Middle 1/3rd
|
67 (24.9) |
28 (25.5) |
39 (24.5) |
|
Upper 1/3rd
|
6 (2.2) |
0 |
6 (3.8) |
|
Tumor size (mm) |
20.7±9.6 |
20.1±9.5 |
21.2±9.6 |
0.356 |
|
Differentiation |
|
|
|
0.365 |
|
Differentiated |
175 (65.1) |
68 (61.8) |
107 (67.3) |
|
Undifferentiated |
94 (34.9) |
42 (38.2) |
52 (32.7) |
|
Depth of invasion |
|
|
|
0.802 |
|
pT1 |
252 (93.7) |
102 (92.7) |
150 (94.3) |
|
pT2 |
9 (3.3) |
5 (4.5) |
4 (2.5) |
|
pT3 |
4 (1.5) |
1 (0.9) |
3 (1.9) |
|
pT4 |
4 (1.5) |
2 (1.8) |
2 (1.3) |
|
Nodal metastasis |
|
|
|
0.525 |
|
pN0 |
247 (91.8) |
103 (93.6) |
144 (90.6) |
|
pN1 |
13 (4.8) |
3 (2.7) |
10 (6.3) |
|
pN2 |
5 (1.9) |
3 (2.7) |
2 (1.3) |
|
pN3 |
4 (1.5) |
1 (0.9) |
3 (1.8) |
|
pTNM stage |
|
|
|
0.848 |
|
I |
253 (94.1) |
103 (93.6) |
150 (94.3) |
|
II |
10 (3.7) |
5 (4.5) |
5 (3.1) |
|
III |
6 (2.2) |
2 (1.8) |
4 (2.5) |

Operative outcomes
Table 2 summarizes the operative outcomes in the 2 groups. Reconstruction techniques showed significant differences between the groups. Most patients (84.4%) in the RPG group underwent intracorporeal anastomosis, whereas most of them (99.1%) in the CPG group underwent extracorporeal anastomosis. Accordingly, Billroth-II or Roux-en-Y gastrojejunostomy was performed more frequently in the CPG group. D2 lymph node dissection was performed more frequently in the RPG group than in the CPG group (54.1% vs. 28.2%; P<0.001). Of the 159 patients in the RPG group, 15 (9.4%) required an additional port insertion during the operation. In 12 patients, 1 trocar was inserted as D2 dissection was difficult with only 3 ports. In 2 of the remaining 3 patients, 1 trocar was inserted owing to a protruding pancreatic body, while in the third patient, it was inserted for adhesiolysis. There was no significant difference in the rate of combined resection between the groups.
Table 2
Operative results

|
Variables |
CPG (n=110) |
RPG (n=159) |
P |
|
Reconstruction |
|
|
<0.001 |
|
Extracorporeal |
109 (99.1) |
23 (15.6) |
|
Intracorporeal |
1 (0.9) |
136 (84.4) |
|
Anastomosis |
|
|
<0.001 |
|
B-I |
82 (74.5) |
19 (11.9) |
|
B-II |
28 (25.5) |
130 (81.9) |
|
Roux-en-Y |
0 |
10 (6.3) |
|
Lymph node dissection |
|
|
<0.001 |
|
D1+ |
79 (71.8) |
73 (45.9) |
|
D2 |
31 (28.2) |
86 (54.1) |
|
Combined resection |
|
|
0.776 |
|
No |
104 (94.5) |
149 (93.7) |
|
Yes |
6 (5.5) |
10 (6.4) |
|
Operative time (min) |
195.9±44.2 |
206.2±39.8 |
0.140 |
|
Operative bleeding (mL) |
56.3±53.2 |
48.2±36.4 |
<0.001 |
|
No. of retrieved lymph nodes |
28.5±11.2 |
34.2±15.3 |
0.001 |

The operative time in the RPG group did not significantly differ from that in the CPG group. However, intraoperative bleeding was significantly lower in the RPG group (56.3 vs. 48.2 mL; P<0.001). The number of retrieved lymph nodes was greater in the RPG group than in the CPG group (34.2 vs. 28.5; P=0.001).
Postoperative outcomes
Table 3 shows the postoperative outcomes in the 2 groups. There were no significant differences between the groups with regard to the first flatus time or length of hospital stay. Patients in the RPG group resumed diet significantly earlier than those in the CPG group did (2.0 vs. 2.6 days; P=0.012). After surgery, 18 patients (16.4%) in the CPG group and 27 patients (17%) in the RPG group developed complications; this difference was not statistically significant. No postoperative deaths occurred in either group. Neither the incidence nor the severity of each complication significantly differed between the groups. Four patients in the CPG group experienced severe complications; 2 patients experienced duodenal stump leakage requiring re-operation, one patient experienced chyle leakage requiring drainage for more than 7 days; and one patient experienced renal failure requiring dialysis. Three patients in the RPG group experienced severe complications; 1 patient each required pigtail insertion for duodenal stump leakage, percutaneous aspiration for intraabdominal infection, and re-operation owing to bleeding.
Table 3
Postoperative outcomes

|
Variables |
CPG (n=110) |
RPG (n=159) |
P |
|
First flatus (day) |
2.7±0.9 |
2.9±0.8 |
0.089 |
|
Diet resumption (day) |
2.5±2.6 |
2.0±0.4 |
0.012 |
|
Hospital stay (day) |
8.8±7.3 |
7.9±4.0 |
0.233 |
|
Morbidity |
18 (16.4) |
26 (17.0) |
0.894 |
|
Severity of complication*
|
|
|
0.075 |
|
Mild |
8 (7.3) |
21 (13.2) |
|
|
Gastric stasis |
5 |
5 |
|
|
Ileus |
0 |
6 |
|
|
Intrabdominal infection |
1 |
3 |
|
|
Ascites |
0 |
3 |
|
|
Bleeding |
1 |
0 |
|
|
Chyle leak |
0 |
1 |
|
|
Wound |
0 |
1 |
|
|
Medical |
1 |
2 |
|
Moderate |
6 (5.5) |
2 (1.3) |
|
|
Gastric stasis |
4 |
0 |
|
|
Ascites |
1 |
1 |
|
|
Bleeding |
1 |
0 |
|
|
Medical |
0 |
1 |
|
Severe |
4 (3.6) |
3 (1.9) |
|
|
Stump leak |
2 |
1 |
|
|
Intrabdominal infection |
0 |
1 |
|
|
Bleeding |
0 |
1 |
|
|
Chyle leak |
1 |
0 |
|
|
Medical |
1 |
0 |
|
Death |
0 |
0 |

Learning curve
The CUSUM learning curve and operative times are shown in
Fig. 2. The CUSUM learning curve consisted of three phases: phase I (the initial 17 cases), phase II (the middle 15 cases), and phase III (the final 53 cases). From the 1st case to the 17th case (phase I), the CUSUM curve showed a rise, followed by a fall from the 18th case to the 33rd case (phase II). After the 34th case (phase III), the curve showed a relatively gentle and flat shape.
 | Fig. 2
Operative time and CUSUM curves of RPG. Green lines indicate breakthrough points (17th and 33rd case).
CUSUM = cumulative sum; RPG = reduced-port gastrectomy.

|
DISCUSSION
With the advances in surgical techniques and instruments, experienced gastric surgeons are increasingly adopting reduced-port or single-port laparoscopic gastrectomy to treat gastric cancer [
89]. However, gastric surgeons with lesser experience use this technique less often owing to concerns about technical difficulties and increased postoperative complications. In the present study, a single surgeon in a small-volume center transitioned from conventional laparoscopic surgery to reduced-port laparoscopic gastrectomy. We found that the operative outcomes of reduced-port laparoscopic gastrectomy, including morbidity and mortality, were similar to those of conventional laparoscopic surgery. The learning curve was also relatively short, at around 30 cases. Therefore, we believe that reduced-port laparoscopic gastrectomy can be adopted safely without increasing patient risk, even in a small-volume center.
In our experience, 3-port laparoscopic gastrectomy has several advantages. Firstly, the operator and laparoscopist can operate without an assistant. Secondly, reduced-port laparoscopic gastrectomy can be performed using conventional abdominal ports and laparoscopic devices, making it more accessible and economical than single-port gastrectomy is. Finally, reduced-port laparoscopic gastrectomy can be performed in the same manner as conventional laparoscopic surgery and shows no significant difference from CPG in terms of operative outcomes. Therefore, reduced-port laparoscopic gastrectomy may generally be more acceptable than single-port gastrectomy for gastric surgeons with less experience.
The feasibility and safety of reduced-port laparoscopic gastrectomy have been reported in previous studies. Jeong et al. [
8] and Kim et al. [
9] compared “duet-LDG” using three abdominal ports with conventional LDG and showed comparable short-term outcomes between the procedures. However, these studies were from large-volume centers with extensive experience of laparoscopic gastrectomy, which may limit the generalizability of the results. In contrast, the present study involved a relatively inexperienced surgeon who had performed about 60 laparoscopic gastrectomies per year before the study. As observed in previous studies, the current study showed that reduced-port surgery had postoperative outcomes (first flatus, hospital stay, and morbidity) that were comparable to those of conventional laparoscopic surgery.
In the present study, extracorporeal anastomosis was performed significantly more often in the CPG group, while intracorporeal anastomosis was performed more often in the RPG group. With our increasing experience of laparoscopic surgery, we gradually adopted intracorporeal Billroth-II or Roux-en-Y anastomosis to eliminate the need for mini-laparotomy for all patients from February 2015. The frequency of D2 dissection was higher in the RPG group. In this study, D2 dissection was more frequently performed in the RPG group despite similar tumor stages of the patients in the 2 groups. As we gained greater proficiency in laparoscopic lymph node dissection over the study period, D2 dissection was more frequently performed for patients with suspicious enlarged lymph nodes. However, the final pathological examination showed similar pathological stages between the groups.
In the present study, the operative time for the RPG group was not significantly different from that for the conventional laparoscopic surgery group; however, significantly less operative blood loss was observed in the RPG group. In this regard, although reduced-port laparoscopic gastrectomy is slightly more difficult to perform without an assistant, the operative time did not significantly increase, probably because the surgeon had accumulated experience of laparoscopic gastrectomy over time. This may also account for the lower blood loss in the RPG group. Nevertheless, reduced-port surgery is not inferior to conventional surgery in terms of operative results. Therefore, the clinical advantages of reduced-port laparoscopic gastrectomy must be further investigated in a randomized controlled trial.
In the present study, the learning curve was evaluated using the CUSUM method. Jeong et al. [
8] reported that experience of at least 30 cases was required to obtain technical expertise in duet-LDG. We observed a tendency toward increased cumulative operative time during the initial 17 operations, and a decrease thereafter. When the operator reached the third phase after 33 operations, the CUSUM of the operative time assumed a gentle plane. This implies that although the mean operative time was similar between the three phases, it stabilized with the experience of the surgeon, reflecting technical proficiency. Meanwhile, other studies on CPG showed the requirement of experience with more cases to overcome the learning curve, as observed in our study [
16]. Therefore, we also believe that an experience of approximately 30 cases is required to acquire technical proficiency in reduced-port laparoscopic gastrectomy.
The present study has some limitations owing to its retrospective nature and the involved selection bias resulting from non-random assignment of operative techniques. However, the study analyzed experience from a single surgeon, which may have ensured better quality control of the surgical technique, although it may also have weakened the generalizability of the results, particularly, the learning curve. Therefore, the learning curve of this technique needs to be investigated in a larger series comprising multiple surgeons with various expertise levels.
In conclusion, the present study showed that the surgical outcomes of reduced-port laparoscopic gastrectomy were comparable to those of CPG in a small-volume center, suggesting that the transition from conventional to reduced-port laparoscopic gastrectomy may be feasible and safe, with a relatively short learning curve for surgeons with less experience. The clinical benefits of reduced-port laparoscopic gastrectomy must be validated in a large, multicenter, randomized clinical trial.








 PDF
PDF ePub
ePub Citation
Citation Print
Print



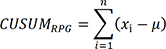

 XML Download
XML Download