INTRODUCTION
Most cancer experts believe that adequate lymph node (LN) dissection provides a survival benefit in the treatment of gastric cancer [
1]. However, multiple vasculatures with complex lymphatic channels within the stomach anatomy increase the difficulty in performing a radical gastrectomy without postoperative morbidity [
2]. Because a high level of proficiency is required in performing radical LN dissection, additional identification of potentially involved LNs may improve surgical outcomes [
3].
We previously reported that injecting diluted indocyanine green (ICG) followed by near-infrared (NIR) imaging was clinically applicable for identifying LNs adjacent to the injection site in a preclinical animal model [
4]. ICG injection aided not only in the detection of LNs but also in the detection of lymphatic channels; thus, it is a method with low toxicity and hypersensitivity that could help identify the complex anatomy of the perigastric lymphatic structures [
4]. Although ICG showed rapid and extensive dispersion with adequate titration and quantification, a NIR-facilitated view can be transmitted to the operating monitor to allow the identification of anatomical structures only where the dye is present.
The aim of this study was to assess the feasibility of NIR imaging with ICG injection in investigating the completeness of laparoscopic LN dissection for gastric cancer.
MATERIALS AND METHODS
Patients
Patients scheduled for laparoscopic gastrectomy as a primary treatment of gastric cancer at Seoul National University Hospital between January 2015 and March 2016 were considered eligible for the study. All surgical procedures were performed in accordance with the preoperative diagnosis regardless of the findings on the NIR view. This study was approved by the Institutional Review Board of Seoul National University Hospital, Seoul, Korea (approval No. 1410-142-622). Written consent was obtained from all patients before any procedure. All procedures were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration.
Study design
A prospective nonrandomized study was designed to investigate the additional role of NIR imaging with ICG in gastric surgery. With this novel technique, the completeness of LN dissection was assessed in 2 steps. First, it was applied in the assessment of the infra-pyloric LN station (#6 LN), as it is considered the most challenging step in node dissection in gastric cancer surgery [
5]. In this step, the same number of patients undergoing laparoscopic pylorus-preserving gastrectomy (LPPG) and those undergoing laparoscopic distal gastrectomy (LDG) were enrolled, and the completeness of LN station #6 was reviewed. To focus on LN station #6, ICG was injected at 4 sites (anterior, posterior, lesser, and greater curvature) in the wall of the antrum through intraoperative endoscopy followed by laparoscopic dissection under white light. After the dissection, the infra-pyloric area was observed using NIR imaging. Additionally, ICG-stained tissues (6E) were resected and sent for pathologic review in the same manner.
In the second step, all stations involved in proper LN dissection (D1+ or D2) for distal gastrectomy (1, 3, 4sb, 4d, 5, 6, 7, 8, 9, and/or 11p, 12) were studied. ICG was injected at 3 sites (antrum, angle, and low body) along the lesser curvature and at 3 sites of the greater curvature that were contralateral to the injection sites of the lesser curvature. After performing LN dissections in all the intended stations, the resected stations were reviewed using NIR imaging. Additional residual soft tissues (E) with ICG uptake at the dissected stations were resected and sent for pathologic evaluation.
Preoperative workup
The diagnosis in all patients was based on pathologic confirmation from endoscopic biopsy of the primary tumor. Endoscopic ultrasound and computerized tomography with a stomach insufflation protocol was performed in all cases for preoperative staging. At 1 or 3 days before surgery, all patients underwent endoscopy for preoperative endoscopic clipping, performed by gastroenterologists, to mark the lesions of early gastric cancer. Endoscopic clipping is the conventional method to mark the location of the tumor in our practice, and it is performed for patient safety as a salvage procedure to facilitate intraoperative endoscopy and as a landmark to avoid while performing submucosal ICG injection.
ICG
The injection procedure used in this study was based on the results of a previous preclinical study [
4]. In that study, the optimal setting for ICG preparation for LN biopsy was injecting 1 mL of a 0.05–0.1 mg/mL solution into 4–5 sites. In our study, 2.5 mg/mL ICG solution (Diagnogreen Inj; Daiichi Sankyo Propharma Co. Ltd., Osaka, Japan) was diluted with 0.9% normal saline and was prepared at a dose of 0.05 mg/mL. The solution (1 mL) was injected at each site 15 minutes before dissection.
Surgical procedure and NIR system
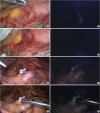
A laparoscopic system with NIR functionality (Karl Storz GmbH & Co. KG, Tuttlingen, Germany) was used in this study. The conversion from white light to the ICG mode was accomplished using a foot pedal. The images were generated using a full high-definition camera system (IMAGE1 S™ NIR-ICG system; Karl Storz GmbH & Co. KG) connected to a laparoscope with a 10-mm, 30° scope (Hopkins telescope; Karl Storz GmbH & Co. KG). After activating the NIR mode, white light was deactivated, and NIR vision was turned on, creating a NIR fluorescence imaging (NIR-ICG) mode (
Figs. 1 and
2).
 | Fig. 1
View of the infra-pyloric area 5 minutes after endoscopic submucosal injection of (4 sites of the antrum): (A) under white light and (B) in NIR mode.
ICG = indocyanine green; NIR = near-infrared.

|
 | Fig. 2
Bidirectional dispersion of fluorescence after endoscopic submucosal injection of ICG on the anterior wall of the stomach: (A) under white light and (B) in NIR mode.
ICG = indocyanine green; NIR = near-infrared.

|
In all patients, 15 minutes after the gastroscopic submucosal injections, the NIR mode was activated to confirm the quality of the ICG injection and to identify the diffusion into the lymphatic channels.
The NIR mode was turned off during the laparoscopic dissection phase. After node dissections were completed, the NIR mode was activated, and each node station was closely reviewed. Every additional tissue with ICG uptake was collected and sent for histologic confirmation. The criterion for tissue collection was a brighter appearance of the tissue in the NIR mode during the laparoscopic view.
All surgical procedures were performed based on the Japanese Gastric Cancer Treatment Guidelines, and ex vivo LN dissections were performed according to the definition of gastric carcinoma based on the Japanese classification [
67].
Data collection
Demographic, surgical, clinicopathologic, and clinical outcomes were recorded. The duration of intraoperative gastroscopy was recorded as the time from intubation to extubation of the gastroscope. All postoperative complications were recorded and graded using the Clavien-Dindo classification. Any findings that were considered an adverse event related to ICG injection were collected and recorded.
The chi-square was calculated, and Student's t-test was used for comparison of means. All tests were 2 sided, and P-values of <0.05 were considered significant. All statistical analyses were performed using IBM SPSS Statistics version 21 (IBM Inc., Chicago, IL, USA).
DISCUSSION
This study aimed to investigate the clinical application of a NIR camera system for radical laparoscopic gastrectomy performed by experienced surgeons at a high-volume center. The current study reports the experience of one the earliest and largest adaptation of the NIR-ICG technique in laparoscopic LN dissection for gastric cancer.
Complete dissection of the infra-pyloric area is technically challenging owing to anatomical variations in the vasculature and conjoining of embryologic planes, whereas nodal metastasis to the right gastroepiploic vessels is frequent in lower- and middle-third gastric cancer [
58]. Pylorus-preserving gastrectomy has a higher likelihood of leaving behind soft tissues after dissection than distal gastrectomy and removing tissues from the small branches of the artery and vein while preserving them requires a higher level of surgical skill [
910]. Recently, intraoperative vascular imaging with the ICG fluorescence technique has been introduced to overcome this hurdle [
11]. With this background, we reported the completeness of LN dissection of the infra-pyloric area during LPPG in addition to that during LDG. Although tissues with ICG uptake were more frequent in LPPG than in LDG, it failed to show a statistical difference.
One of the limitations of our study was a high false-positive rate. Among 15 additional tissue specimens with ICG uptake, only 2 actual LNs and 13 visceral adipose specimens were obtained. The high temperature of the energy device or forceful handling of the LNs might have compromised the nodal structure, causing its deformity. Owing to false positivity under this setting and because unnecessary soft tissue dissection in patients with early gastric cancer may lead to additional injury, cautious application is required.
Moreover, compared with previous modalities, the NIR camera can detect even a very small fraction of ICG from the operation field, which might be confusing in the early application of this technique. To overcome this issue, additional quantification of the ICG signal could provide complementary information to distinguish tissues with different levels of fluorescence uptake. A quantification method can offset the false-positive ICG tissues and deliver precise information about the targeted tissues.
It was difficult to consider the sample size of this study for 2 reasons. First, this was an investigational study evaluating the feasibility of the NIR-ICG technique in various partial gastrectomy settings [
12]. Second, although various methods have been proven safe and useful in gastric cancer mapping, there are no reports about the completeness of LN dissection with the NIR-ICG technique, which made it difficult to calculate the number of samples required for the anticipated events to develop [
131415]. Because the studied procedure was not performed or reported before the time of patient enrolment, it was difficult to follow the conventional sample size calculation. To compensate for this problem, we considered that this study should be categorized as an Innovation, Development, Exploration, Assessment, Long-term (IDEAL) phase 2a study, as introduced in previous publications about surgical [
1617].
Randomization could have provided stronger evidence for this study; however, the current protocol of the NIR-ICG technique still requires further revision, and a randomized clinical trial should be conducted after more background data are collected about this technique and after establishment of a more sophisticated protocol [
18].
In our experience, the key factor for successful lymphatic mapping was the delivery of the tracer. The concentration and amount of injection are associated with the timing and degree of dispersion. The correct concentration and dosage should be calibrated according to the design and purpose of the study. We adopted the concentration at the lowest limit of our preclinical study (0.05 mg/mL) to obtain an optimal outcome from multiple (4–6) injections along the stomach. Second, the submucosal injection skill is important. Deep injection causes intravascular injection, which results in total blurriness of adjacent organs. In cases of extraluminal spillage, even small amounts can contaminate the operation field. Meanwhile, when ICG was injected only within the surface of the mucosa, a lag time in the perfusion of ICG into the laminal flow of the lymphatic system was noted, making it difficult to determine whether the procedure was successful.
In this study, we had to exclude 1 case. Initially, we proceeded with the operation; however, during dissection along the pancreas, we found a serious vascular anomaly: the portal vein was running through the anterior surface of the pancreas head and across the second part of the duodenum, and the common hepatic artery was totally replaced by an aberrant hepatic artery from the left gastric artery. For the safety of the patient, we decided to drop the patient from the study, and the NIR camera was turned on during the rest of the operation. With ICG-enhanced fluorescence until the end of the operation, we were able to perform a rather comfortable and safe surgery owing to a virtual lymphangiogram, which allowed the surgeon to differentiate the lymphatic structures from other anatomical structures and avoid injury of the unfamiliar vascular anatomy. In this case, the additional visual information provided a higher level of confirmation for the operator to avoid potential injury and bleeding when performing the D2 dissection, which could be helpful even for highly experienced gastric surgeons. This NIR technique can provide guidance in dissecting lymphatic tissues with safe visual confirmation and can improve intraoperative decision making during complex lymphatic dissections.
In summary, this report showed the feasibility and efficacy of an NIR camera system in laparoscopic gastrectomy for gastric cancer, which was facilitated by our experience in LN mapping. The quality of the images depends on the preparation of the tracer and the quality of its injection. In our experience, the benefit of this technique seemed limited to standardized laparoscopic radical partial gastrectomy cases with a normal anatomy.
The clinical implication of this study is that even for surgeons with a high level of experience in laparoscopic D2 dissections, the NIR system can serve as a complimentary tool to confirm complete LN dissection in patients with atypical anatomy. Meanwhile, for inexperienced surgeons, to perform function-preserving surgeries, such as LPPG, additional confirmation of complete dissection should benefit the surgical procedure to overcome the limitation of insufficient LN dissection.










 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download