Abstract
Purpose
Intestinal dysfunction is one of the most common complications in patients after abdominal surgery. Daikenchuto (DKT), a traditional herbal medicine, is recently employed to improve postoperative intestinal dysfunction. The aim of this meta-analysis was to assess the efficacy of DKT in improving intestinal dysfunction after abdominal surgery.
Methods
PubMed, Embase, and the Cochrane library were systematically searched to identify randomized controlled trails (RCTs) in adult patients undergoing abdominal surgery, who were randomly distributed to administrate DKT and placebo. The primary outcomes included the time to first postoperative flatus or bowel movement. We used random-effects models to calculate summary mean differences (MDs) with 95% confidence intervals (CIs).
Results
Nine RCTs totaling 1,212 patients (618 in DKT, 594 in control group) were included in our study. Compared with control group, DKT can effectively improve postoperative intestinal dysfunction by shortening the time to first postoperative flatus (MD, −0.41; 95% confidence interval [CI], −0.66 to −0.16; P = 0.001) with significant heterogeneity (I2 = 71%, P = 0.004), and bowel movement (MD, −0.65; 95% CI, −0.97 to −0.32; P < 0.001) without significant heterogeneity (I2 = 40%, P = 0.14). Sensitivity analyses by indication of surgery and type of surgery yielded similar results.
Conclusion
These data provide limited evidence that DKT shows efficacy on improving intestinal dysfunction after abdominal surgery. However, the results should be interpreted cautiously, due to the heterogeneity of the studies included. Thus, the efficacy of DKT on improving postoperative intestinal dysfunction warrants further investigation.
Intestinal dysfunction after abdominal surgery is the leading cause of prolonged hospital stays and additional health-care costs, which has been considered a common and inevitable outcome to some extent [1]. Thus, improving postoperative intestinal dysfunction is of great importance. It is characterized by abdominal distention, nausea, vomiting, delayed passage of flatus or bowel movement. In recent decades, many therapeutic strategies have been used to improve postoperative intestinal dysfunction, such as fast track surgery [2]. However, all have limited efficacy and are not free of side effects.
Daikenchuto (DKT) is one of the most widely administered traditional herbal medicines in the Asia-Pacific area with active components containing Japanese pepper, processed ginger, and ginseng [3]. It has been recently employed to improve intestinal dysfunction [4] and very few side effects have been reported. In intestinal ischemia-related diseases, DKT was associated with ameliorating microvascular dysfunction [5]. Several studies demonstrate that DKT significantly increases superior mesenteric artery blood flow resulting in improvement of intestinal dysfunction [6]. However, the studies regarding DKT in improving postoperative intestinal dysfunction have conveyed inconclusive results [789101112131415]. We therefore conducted a meta-analysis of relevant randomized controlled trials to assess the efficacy of DKT in improving intestinal dysfunction after abdominal surgery.
This meta-analysis followed the Cochrane Handbook for Systematic Reviews of Interventions [16] and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary material 1) [17]. Two reviewers separately conducted literature retrieval, data extraction, quality assessment, and statistical analysis, with inconsistency solved by discussion and by the chief reviewer. Specially, a statistician in our group performed and reviewed the statistical section.
Databases (PubMed, the Cochrane Library, and Embase) were comprehensively searched to identify relevant trials (up to February 10, 2017). The search strategy used the following format terms: “Daikenchuto” or “Dai-kenchu-to” or “Dai-ken-chu-to” or “DKT” or “TJ-100” or “N100” or “TU-100”. DKT, TJ-100, N100, and TU-100 are the trade names of Daikenchuto. No language restriction was imposed. Moreover, reference to relevant articles was examined manually for potentially eligible trials. The whole search process was carried out iteratively until no new publications could be found. Detailed search strategies were attached to Supplementary material 2. We present an adapted PRISMA flowchart showing the process of study selection. We only included randomized controlled trails (RCTs) focused on DKT for intestinal dysfunction in patients after abdominal surgery. Detailed inclusion and exclusion criteria are presented in Table 1.
The following data would be extracted: first author, year of publication, clinical setting, demographic feature (number of patients, age, and sex), type of interventions for DKT, indication of surgery, type of surgery and outcomes, which would be entered into a normalized data collection Excel form (Microsoft Corp., Redmond, WA, USA). Quality assessment was performed by using the guidelines from the Cochrane tool [18]. All included studies were assigned as ‘low,’ ‘unclear’ or ‘high’ risk, using the following criteria: (1) random, (2) allocation concealment, (3) blinding, (4) incomplete outcome data, (5) selective reporting, and (6) other bias by 2 independent investigators.
We evaluated the efficacy of DKT on improving postoperative intestinal dysfunction based on the data from included RCTs. The time to first postoperative flatus and the time to first postoperative bowel movement were treated as continuous variables and presented as mean difference (MD) with 95% confidence interval (CI). We calculated standard deviations with standard formulae if only medians and ranges were provided. For studies that had not shown the corresponding results, we extracted data from the Kaplan-Meier curves with the Engauge Digitizer version 4.1 (M Mitchell, Engauge Digitizer; http://markummitchell.github.io/engauge-digitizer) [1920]. Heterogeneity was estimated by using the I2 statistic, a quantitative measure of inconsistency [21]. I2 of less than 50% was regarded as accepted heterogeneity. In cases of significant heterogeneity, a random-effects model was used. Whether heterogeneity was present, sensitivity analyses were conducted to evaluate the robustness of our results. Sensitivity analyses were done according to indication of surgery and type of surgery. In addition, the ‘leave-one-out’ influence analyses were performed to explore the influence of a single study on overall pooled effect estimate by omitting a study each time.
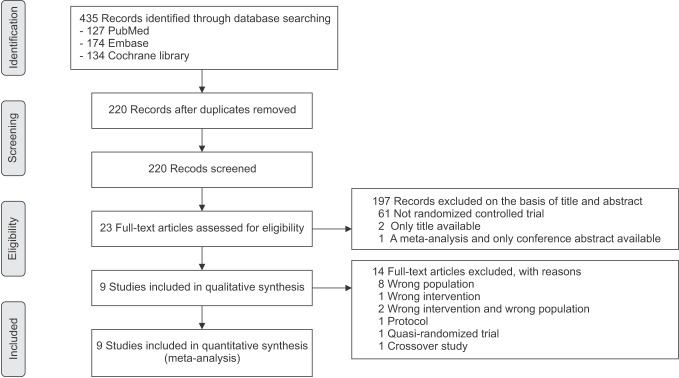
The initial search identified 435 relevant publications. After excluding duplicate studies (n = 214) and publications not relevant to DKT (n = 133), not RCT (n = 61), only title available (n = 2), only conference abstract available (n = 1), 23 studies were reviewed for full text. Apart from protocols (n = 1), wrong population and/or wrong intervention (n = 11), we also eliminated a quasi-randomized trial and a crossover study. For a detailed description, see Supplementary material 3. Finally, 9 RCTs [789101112131415] met inclusion criteria and were included in the final analysis. The selection process is shown in Fig. 1.
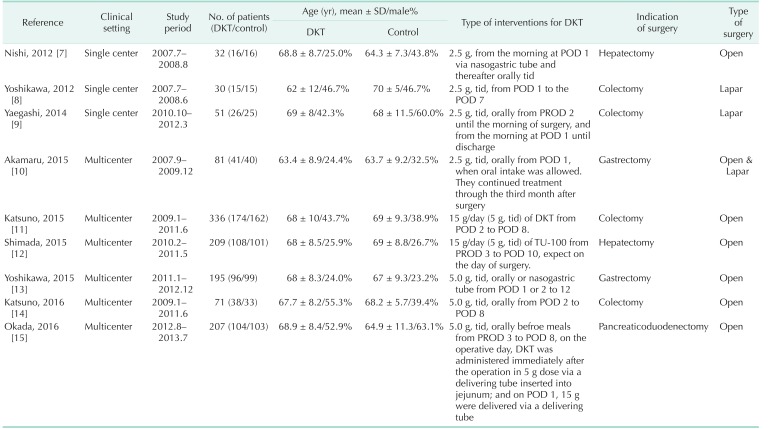
Table 2 summarizes the main feature of the nine RCTs, which were published from 2012 to 2016. The individual total sample sizes ranged from 30 to 336 (total of 1,212 participants). Six hundred eighteen participants were randomized to DKT and 594 to control group. Among these nine RCTs, 6 reported the time to first postoperative flatus [789131415]; 6 reported the time to first postoperative bowel movement [7910111213]. Two studies [89] were about laparoscopic surgery and the others [71112131415] on open procedures. Akamaru et al. [10] reported on laparoscopic and open surgery. Four trials [891114] reported colectomy.
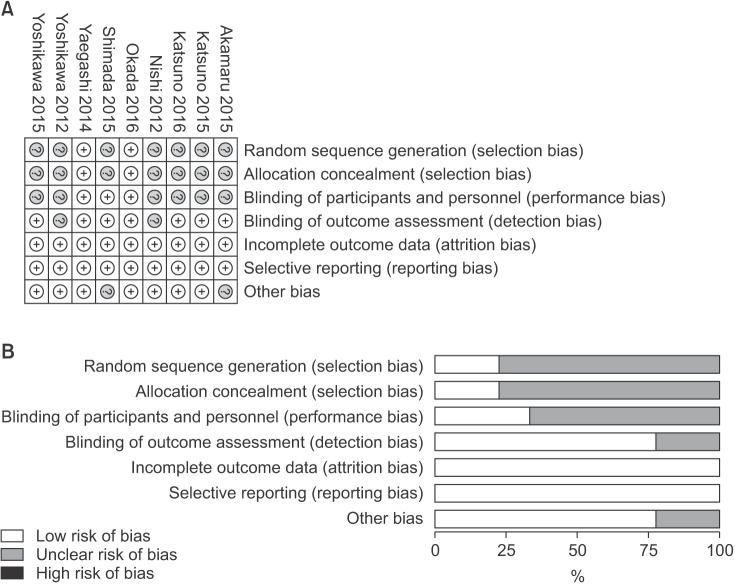
Risk-of-bias details for individual trials were exhibited in Fig. 2A, and the summary of risk of bias in Fig. 2B. Appropriate randomization was produced to avoid possible selection bias in 2 studies [915], which also concealed the allocation sequence using reasonable methods. Two studies [1012] reported their source of funding. As a whole, 2 studies were low risk of bias [915] and others unclear [781011121314].
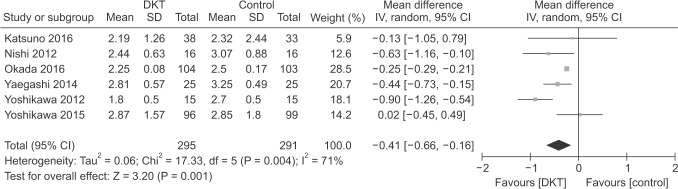
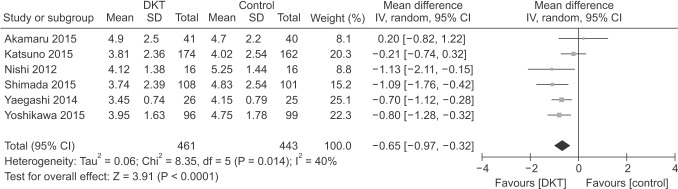
As shown in Figs. 3 and 4, DKT was associated with significantly improving postoperative intestinal dysfunction compared to control group (P < 0.05). Six included studies [789131415] totaling 586 patients (295 in DKT group, 291 in control group) reporting the time to first postoperative flatus. The overall effect favored DKT group (MD, −0.41; 95% CI, −0.66 to −0.16; P = 0.001) (Fig. 3), with significant heterogeneity (I2 = 71%; P = 0.004) (Fig. 3). Moreover, compared with control group, DKT was associated with shortening the time to first postoperative bowel movement (MD, −0.65; 95% CI, −0.97 to −0.32; P < 0.0001) (Fig. 4), without significant heterogeneity (I2 = 40%, P = 0.14) (Fig. 4).
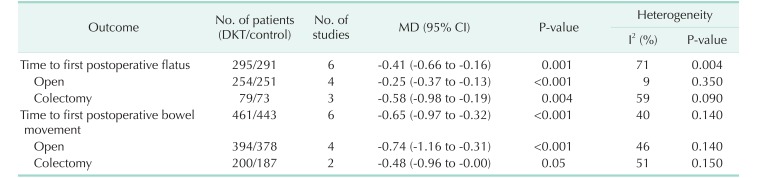
Subsequently, sensitivity analyses were performed to explore the underlying source of heterogeneity and examine the influence of various inclusion criteria on the pooled estimates. The sensitivity analysis in Table 3 based on different inclusion criteria also indicated that DKT was associated with significantly improving postoperative intestinal dysfunction. Inclusion studies of open surgery yielded similar results in the time to first postoperative flatus (4 RCTs; MD, −0.25; 95% CI, −0.37 to −0.13; P < 0.001) (Table 3), with no evidence of heterogeneity (I2 = 9%, P = 0.35), and the time to first postoperative bowel movement (3 RCTs; MD, −0.74; 95% CI, −1.16 to −0.31; P < 0.001) (Table 3), without significant heterogeneity (I2 = 46%, P = 0.14) (Table 3). After inclusion studies of colectomy, the results were still maintained in the time to first postoperative flatus (4 RCTs; MD, −0.58; 95% CI, −0.98 to −0.19; P < 0.004) (Table 3), yet heterogeneity was still present (I2 = 59%, P = 0.09) (Table 3). The studies undergoing colectomy were associated with a slightly decreasing trend in the time to first postoperative bowel movement (2 RCTs; MD, −0.48; 95% CI, −0.96 to −0.00; P = 0.05) (Table 3) with heterogeneity (I2 = 51%; P = 0.15) (Table 3). Furthermore, we performed influence analyses. MD for the time to first postoperative flatus maintained a slight fluctuation from −0.48 (95% CI, −0.77 to −0.20] to −0.28 (95% CI, −0.41 to −0.15), and bowel movement −0.76 (95% CI, −1.07 to −0.45) to −0.57 (95% CI, −0.91 to −0.22) after exclusion of any single trial.
To our knowledge, this is the first investigation on the efficacy of DKT on intestinal dysfunction after abdominal surgery using meta-analysis of RCTs methodology. In our meta-analysis, DKT was associated with improving postoperative intestinal dysfunction by shortening the time to first postoperative flatus and bowel movement. This indicates that DKT has benefits and should be available as an approach for patients with postoperative intestinal dysfunction.
The principal finding of our meta-analysis is of clinical value, to some extent. Previous clinical control studies have reported conflicting results, with some showing a decrease in the time to postoperative first flatus and bowel movement [9] and some others demonstrating no effect [14]. What is of note is that the number of participants included in these studies was small, for instance, only 15 per group in Yoshikawa 2012 [8], which makes it difficult to come to a solid conclusion. Obviously, the pooled analysis of RCTs was closer to the true intervention effect, which further reveals the advantage of meta-analysis methodology. Therefore, we used extensive inclusion criteria and included various operations with different characteristics to make the results more clinically useful and generalisable.
The current available evidence of our meta-analysis suggested that DKT can be effectively and safely used to improve intestinal dysfunction in patients after abdominal surgery. Moreover, regardless of difference, sensitivity analyses further confirmed the creditability of the pooled intervention effect in outcome of the time to first postoperative flatus. Laparoscopy surgery has many potential advantages in earlier gastrointestinal recovery and shorter hospital stays over conventional open surgery [2425]. However, the results of sensitivity analysis for type of surgery showed that studies of open surgery showed significant decreases in the time to first postoperative flatus and the time to first postoperative bowel movement. Inclusion studies of colectomy showed a significant decrease in the time to first postoperative flatus and a decreasing trend in the time to postoperative bowel movement. After carefully checking, we found that the number of studies enrolled was very small (2 RCTs), and the results may have been influenced by potential biases. Therefore, further studies should focus on large, well-designed RCTs that focus on this issue.
There is accumulating evidence of DKT providing an important contribution in improving intestinal dysfunction [7891215]. Mechanisms underlying this beneficial effect are not fully understood and are most likely multifactorial. The effects of DKT on intestinal transit or motility might be mediated by cholinergic and 5-hydroxytryptamine mechanisms, as demonstrated by experiments finding that DKT was effective against morphine/chlorpromazine-induced intestinal disorders in rodents [26]. It has been demonstrated that DKT has the ability to reduce inflammatory reaction mediated by alpha7 nicotinic acetylcholine receptors activation [27], inhibit cyclooxygenase-2 activity [28], increase the intestinal blood flow through calcitonin gene-related peptide levels [629], and reverse bacterial translocation [30]. The current understanding of DKT in patients with intestinal dysfunction remains incomplete and well-designed studies are required further.
DKT is one of the most widely administered herbal medicines in Japan, and is mainly used for patients with postoperative ileus. Apparently, this compound is often used in Asia-Pacific countries but not in the West. However, it has been approved by the U.S. Food and Drug Administration as an investigational drug in the United States [12]. Moreover, several clinical trials have been launched to investigate its efficacy for Crohn disease, irritable bowel syndrome, and constipation [15]. DKT is a pharmaceutical-grade extract, which is under strict quality-control criteria, comparable with western pharmaceutical drugs in terms of therapeutic strength. It is expected that DKT will be more acceptable to patients and doctors in future clinical practice.
Several limitations should be taken into account. First, we confirmed the efficacy of DKT, but we included a variety of surgeries and different surgical approaches with inherent clinical and methodological heterogeneity, which may be restricted in some specific types of surgeries and thereby have an impact on our results. For example, pancreaticoduodenectomy is a multi-organ operative procedure with a high incidence of morbidity. Thus, further individual participant data meta-analysis focusing on more homogeneous clinical situations should be conducted. In our present meta-analysis, we made a pragmatic decision that to combine all kinds of surgeries reporting DKT use would be more generalisable to clinical practice than to group specific types. In addition, to mitigate heterogeneity, we used the random-effects model. Second, the current meta-analysis confirmed the effectiveness of DKT on improvement of postoperative intestinal dysfunction, but the dosage, the method, and the duration of administration varied in the included studies. Actually, the optimum DKT dosage, the method of administration, and the duration associated with DKT products are unclear. Accordingly, further studies are warranted to explore this optimization. Finally, the present meta-analysis is based on limited studies.
In summary, our meta-analysis is of clinical significance. The current available results show that DKT can significantly shorten the time to first postoperative flatus and time to first postoperative bowel movement, and improve intestinal dysfunction after abdominal surgery. However, it should be interpreted with caution, because of the significant heterogeneity of the studies. Thus, the efficacy of DKT on improving postoperative intestinal dysfunction warrants further investigation.
ACKNOWLEDGEMENTS
This work was supported by the Natural Science Foundation of China (81302143, 81301865, 81300392, 81672412); the Guandong Natural Science Foundation (2015A030313033); Science and Technology Program of Guangzhou, China (201607010111, 201610010022); the Guangdong Science and Technology Foundation (2016A020215199); Grant [2013]163 from Key Laboratory of Malignant Tumor Molecular Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology; Grant KLB09001 from the Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes; Grant from Guangdong Science and Technology Department (2015B050501004).
References
1. Asgeirsson T, El-Badawi KI, Mahmood A, Barletta J, Luchtefeld M, Senagore AJ. Postoperative ileus: it costs more than you expect. J Am Coll Surg. 2010; 210:228–231. PMID: 20113944.

2. Kehlet H. Surgery: Fast-track colonic surgery and the ‘knowing-doing’ gap. Nat Rev Gastroenterol Hepatol. 2011; 8:539–540. PMID: 21894195.
3. Kono T, Shimada M, Yamamoto M, Kaneko A, Oomiya Y, Kubota K, et al. Complementary and synergistic therapeutic effects of compounds found in Kampo medicine: analysis of daikenchuto. Front Pharmacol. 2015; 6:159. PMID: 26300774.

4. Mochiki E, Yanai M, Ohno T, Kuwano H. The effect of traditional Japanese medicine (Kampo) on gastrointestinal function. Surg Today. 2010; 40:1105–1111. PMID: 21110152.

5. Kono T, Omiya Y, Hira Y, Kaneko A, Chiba S, Suzuki T, et al. Daikenchuto (TU-100) ameliorates colon microvascular dysfunction via endogenous adrenomedullin in Crohn's disease rat model. J Gastroenterol. 2011; 46:1187–1196. PMID: 21808981.

6. Takayama S, Seki T, Watanabe M, Takashima S, Sugita N, Konno S, et al. The effect of warming of the abdomen and of herbal medicine on superior mesenteric artery blood flow - a pilot study. Forsch Komplementmed. 2010; 17:195–201. PMID: 20829597.

7. Nishi M, Shimada M, Uchiyama H, Ikegami T, Arakawa Y, Hanaoka J, et al. The beneficial effects of Kampo medicine Dai-ken-chu-to after hepatic resection: a prospective randomized control study. Hepatogastroenterology. 2012; 59:2290–2294. PMID: 23435143.
8. Yoshikawa K, Shimada M, Nishioka M, Kurita N, Iwata T, Morimoto S, et al. The effects of the Kampo medicine (Japanese herbal medicine) “Daikenchuto” on the surgical inflammatory response following laparoscopic colorectal resection. Surg Today. 2012; 42:646–651. PMID: 22202972.

9. Yaegashi M, Otsuka K, Itabashi T, Kimura T, Kato K, Fujii H, et al. Daikenchuto stimulates colonic motility after laparoscopic-assisted colectomy. Hepatogastroenterology. 2014; 61:85–89. PMID: 24895799.
10. Akamaru Y, Takahashi T, Nishida T, Omori T, Nishikawa K, Mikata S, et al. Effects of daikenchuto, a Japanese herb, on intestinal motility after total gastrectomy: a prospective randomized trial. J Gastrointest Surg. 2015; 19:467–472. PMID: 25564322.

11. Katsuno H, Maeda K, Kaiho T, Kunieda K, Funahashi K, Sakamoto J, et al. Clinical efficacy of Daikenchuto for gastrointestinal dysfunction following colon surgery: a randomized, double-blind, multicenter, placebo-controlled study (JFMC39-0902). Jpn J Clin Oncol. 2015; 45:650–656. PMID: 25972515.

12. Shimada M, Morine Y, Nagano H, Hatano E, Kaiho T, Miyazaki M, et al. Effect of TU-100, a traditional Japanese medicine, administered after hepatic resection in patients with liver cancer: a multi-center, phase III trial (JFMC40-1001). Int J Clin Oncol. 2015; 20:95–104. PMID: 24595550.

13. Yoshikawa K, Shimada M, Wakabayashi G, Ishida K, Kaiho T, Kitagawa Y, et al. Effect of Daikenchuto, a traditional Japanese herbal medicine, after total gastrectomy for gastric cancer: a multicenter, randomized, double-blind, placebo-controlled, phase II trial. J Am Coll Surg. 2015; 221:571–578. PMID: 26141466.

14. Katsuno H, Maeda K, Ohya M, Yoshioka K, Tsunoda A, Koda K, et al. Clinical pharmacology of daikenchuto assessed by transit analysis using radiopaque markers in patients with colon cancer undergoing open surgery: a multicenter double-blind randomized placebo-controlled study (JFMC39-0902 additional study). J Gastroenterol. 2016; 51:222–229. PMID: 26162646.

15. Okada K, Kawai M, Hirono S, Fujii T, Kodera Y, Sho M, et al. Evaluation of the efficacy of daikenchuto (TJ -100) for the prevention of paralytic ileus after pancreaticoduodenectomy: A multicenter, double-blind, randomized, placebo-controlled trial. Surgery. 2016; 159:1333–1341. PMID: 26747224.

16. Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions. version 5.1.0 [Internet]. Oxford (UK): The Cochrane Collaboration;2011. uptated 2011 Mar. cited 2017 Feb 10. Available from: http://handbook-5-1.cochrane.org/.
17. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009; 339:b2535. PMID: 19622551.

18. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928. PMID: 22008217.

19. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007; 8:16. PMID: 17555582.

20. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998; 17:2815–2834. PMID: 9921604.

21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560. PMID: 12958120.

22. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–1101. PMID: 7786990.

23. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634. PMID: 9310563.

24. van Bree SH, Nemethova A, Cailotto C, Gomez-Pinilla PJ, Matteoli G, Boeckxstaens GE. New therapeutic strategies for postoperative ileus. Nat Rev Gastroenterol Hepatol. 2012; 9:675–683. PMID: 22801725.

25. Sheng QS, Pan Z, Chai J, Cheng XB, Liu FL, Wang JH, et al. Complete mesocolic excision in right hemicolectomy: comparison between hand-assisted laparoscopic and open approaches. Ann Surg Treat Res. 2017; 92:90–96. PMID: 28203556.

26. Satoh K, Kase Y, Yuzurihara M, Mizoguchi K, Kurauchi K, Ishige A. Effect of Daikenchu-to (Da-Jian-Zhong-Tang) on the delayed intestinal propulsion induced by chlorpromazine in mice. J Ethnopharmacol. 2003; 86:37–44. PMID: 12686439.

27. Endo M, Hori M, Ozaki H, Oikawa T, Hanawa T. Daikenchuto, a traditional Japanese herbal medicine, ameliorates postoperative ileus by anti-inflammatory action through nicotinic acetylcholine receptors. J Gastroenterol. 2014; 49:1026–1039. PMID: 23846546.

28. Hayakawa T, Kase Y, Saito K, Hashimoto K, Ishige A, Komatsu Y, et al. Effects of Dai-kenchu-to on intestinal obstruction following laparotomy. J Smooth Muscle Res. 1999; 35:47–54. PMID: 10463435.

29. Takayama S, Seki T, Watanabe M, Monma Y, Sugita N, Konno S, et al. The herbal medicine Daikenchuto increases blood flow in the superior mesenteric artery. Tohoku J Exp Med. 2009; 219:319–330. PMID: 19966532.

30. Takasu C, Yismaw WG, Kurita N, Yoshikawa K, Kashihara H, Kono T, et al. TU-100 exerts a protective effect against bacterial translocation by maintaining the tight junction. Surg Today. 2017; 47:1287–1294. PMID: 28421347.

SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://www.astr.or.kr/src/sm/astr-95-7-001.pdf.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download