Abstract
Background/Aims
Diagnostic tests for carcinoembryonic antigen (CEA) in ascites have been performed in various malignant cases, but there is only few data on the applicability of CEA for colorectal cancer (CRC) patients with peritoneal carcinomatosis. We aimed to determine the usefulness of CEA in ascites (aCEA) as a diagnostic parameter for CRC with peritoneal carcinomatosis.
Methods
Between January 2000 and May 2013, the medical records of 259 patients who underwent paracentesis for the evaluation of ascites were retrospectively reviewed. CRC patients with ascites (n=82) and patients with nonmalignant ascites (n=177) were evaluated. Patients who had other malignancies, including gastric or ovarian cancer, with ascites were excluded. The optimal diagnostic cut-off value of aCEA for CRC with peritoneal carcinomatosis was determined using receiver operating characteristic curve analysis. The value of aCEA for predicting the occurrence of peritoneal carcinomatosis was evaluated using a logistic regression model.
Results
The optimal cut-off value of aCEA to diagnose CRC with peritoneal carcinomatosis was 3.89 ng/mL, and the area under the curve for aCEA was 0.996 (sensitivity 96.3%, specificity 100%, positive predictive value 100%, negative predictive value 98.3%). Multivariate logistic regression analysis showed that aCEA was an independent factor predicting the occurrence of peritoneal carcinomatosis.
Go to : 
References
2. Runyon BA, Hoefs JC, Morgan TR. Ascitic fluid analysis in malignancy-related ascites. Hepatology. 1988; 8:1104–1109.

3. Salerno F, Restelli B, Incerti P, et al. Utility of ascitic fluid analysis in patients with malignancy-related ascites. Scand J Gastroenterol. 1990; 25:251–256.

4. Boyer TD, Kahn AM, Reynolds TB. Diagnostic value of ascitic fluid lactic dehydrogenase, protein, and WBC levels. Arch Intern Med. 1978; 138:1103–1105.

5. Kaleta EJ, Tolan NV, Ness KA, O'Kane D, Algeciras-Schimnich A. CEA, AFP and CA 19–9 analysis in peritoneal fluid to differentiate causes of ascites formation. Clin Biochem. 2013; 46:814–818.

6. Paré P, Talbot J, Hoefs JC. Serum-ascites albumin concentration gradient: a physiologic approach to the differential diagnosis of ascites. Gastroenterology. 1983; 85:240–244.

7. Archimandritis A, Kapsalas D, Douvara M, Tjivras M, Tsirantonaki M, Fertakis A. Value of ascitic fibronectin and cholesterol concentration in the differentiation between malignancy-related and nonmalignant ascites. Ann Med Interne (Paris). 1996; 147:145–150.
8. Saif MW, Siddiqui IA, Sohail MA. Management of ascites due to gastrointestinal malignancy. Ann Saudi Med. 2009; 29:369–377.

9. Loewenstein MS, Zamcheck N. Carcinoembryonic antigen (CEA) levels in benign gastrointestinal disease states. Cancer. 1978; 42(3 Suppl):1412–1418.

10. Tuzun Y, Yilmaz S, Dursun M, et al. How to increase the diagnostic value of malignancy-related ascites: discriminative ability of the ascitic tumour markers. J Int Med Res. 2009; 37:87–95.

11. Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002; 89:1545–1550.

12. Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006; 243:212–222.
13. Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000; 88:358–363.
14. Yajima K, Kanda T, Ohashi M, et al. Clinical and diagnostic significance of preoperative computed tomography findings of ascites in patients with advanced gastric cancer. Am J Surg. 2006; 192:185–190.

15. Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006; 106:1624–1633.

16. Shen-Gunther J, Mannel RS. Ascites as a predictor of ovarian malignancy. Gynecol Oncol. 2002; 87:77–83.

17. Burke EC, Karpeh MS Jr, Conlon KC, Brennan MF. Peritoneal lavage cytology in gastric cancer: an independent predictor of outcome. Ann Surg Oncol. 1998; 5:411–415.

18. Vogel P, Rüschoff J, Kümmel S, et al. Prognostic value of microscopic peritoneal dissemination: comparison between colon and gastric cancer. Dis Colon Rectum. 2000; 43:92–100.
19. Gozalan U, Yasti AC, Yuksek YN, Reis E, Kama NA. Peritoneal cytology in colorectal cancer: incidence and prognostic value. Am J Surg. 2007; 193:672–675.

20. Hase K, Ueno H, Kuranaga N, Utsunomiya K, Kanabe S, Mochizuki H. Intraperitoneal exfoliated cancer cells in patients with colorectal cancer. Dis Colon Rectum. 1998; 41:1134–1140.

21. Yamamoto S, Akasu T, Fujita S, Moriya Y. Longterm prognostic value of conventional peritoneal cytology after curative resection for colorectal carcinoma. Jpn J Clin Oncol. 2003; 33:33–37.

22. González-Moreno S, González-Bayón L, Ortega-Pérez G, González- Hernando C. Imaging of peritoneal carcinomatosis. Cancer J. 2009; 15:184–189.

23. Gerbes AL, Jüngst D, Xie YN, Permanetter W, Paumgartner G. Ascitic fluid analysis for the differentiation of malignancy-related and nonmalignant ascites. Proposal of a diagnostic sequence. Cancer. 1991; 68:1808–1814.

24. Nystrom JS, Dyce B, Wada J, Bateman JR, Haverback B. Carcinoembryonic antigen titers on effusion fluid. A diagnostic tool? Arch Intern Med. 1977; 137:875–879.

25. Jung M, Jeung HC, Lee SS, et al. The clinical significance of ascitic fluid CEA in advanced gastric cancer with ascites. J Cancer Res Clin Oncol. 2010; 136:517–526.

26. Lee IK, Kim DH, Gorden DL, et al. Prognostic value of CEA and CA 19–9 tumor markers combined with cytology from peritoneal fluid in colorectal cancer. Ann Surg Oncol. 2009; 16:861–870.

Go to : 
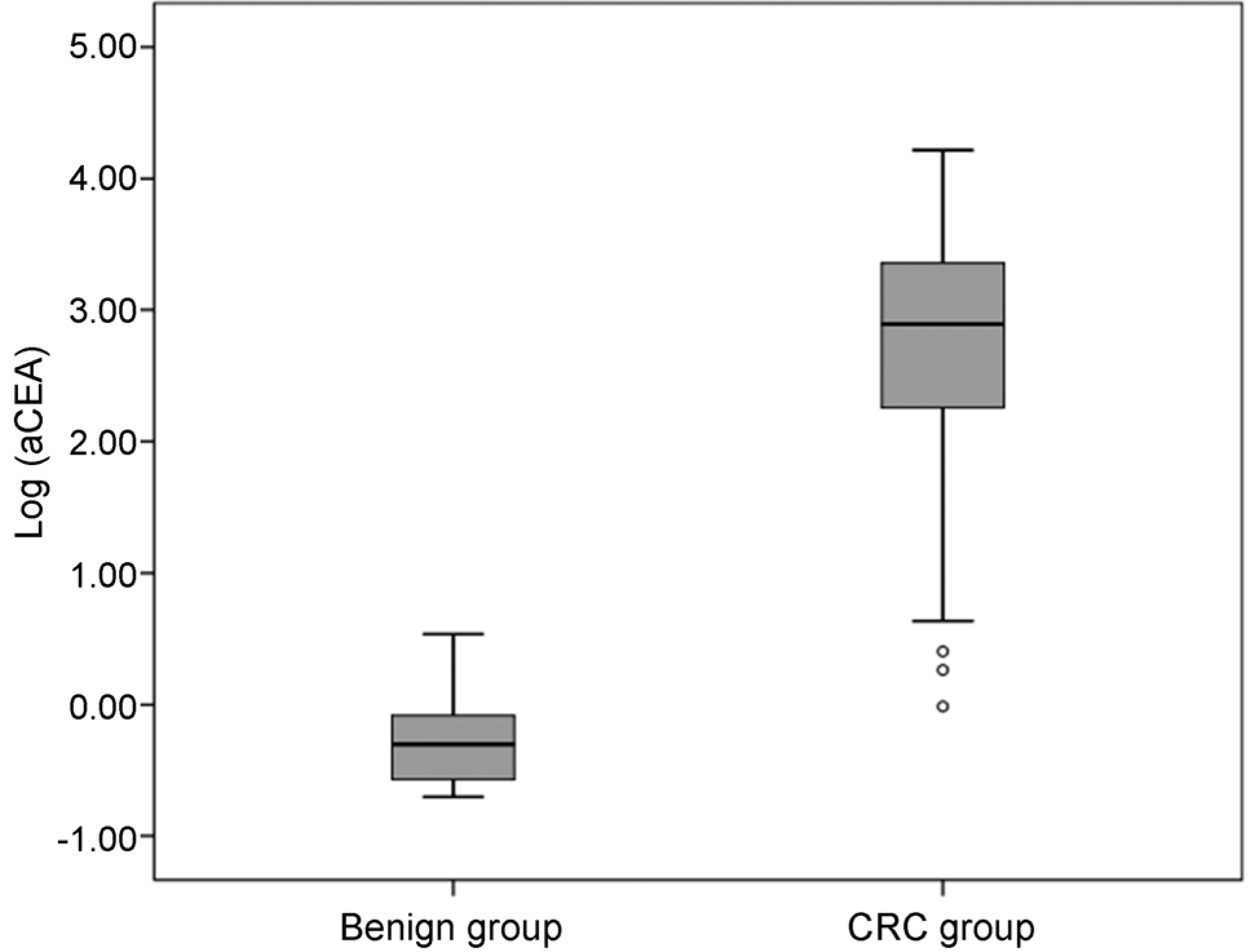 | Fig. 1.Levels of aCEA in the benign and CRC groups. aCEA, carcinoembryonic antigen in ascites; CRC, colorectal cancer. |
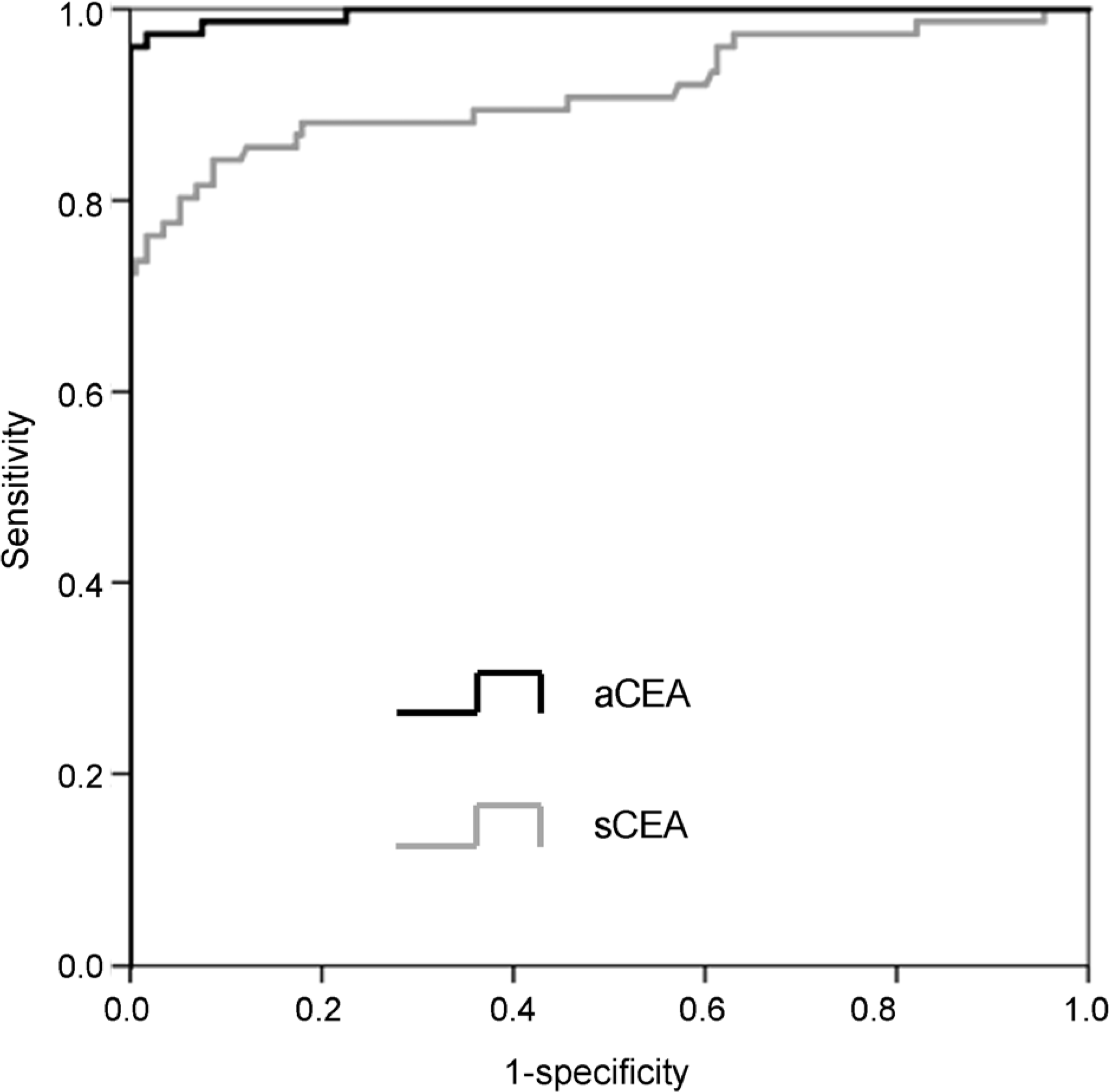 | Fig. 2.ROC curve of diagnostic parameters. ROC, receiver operating characteristic; aCEA, carcinoembryonic antigen in ascites; sCEA, serum carcinoembryonic antigen. |
Table 1.
Baseline Characteristics in Enrolled Patients
| Benign group (n=177) | CRC group (n=82) | |
|---|---|---|
| Median age (range) | 59 (17–87) | 65 (37–47) |
| Gender | ||
| Male | 126 (71.2) | 54 (65.9) |
| Female | 51 (28.8) | 28 (34.1) |
| Performance status a | ||
| 0 | 133 (75.1) | 57 (69.5) |
| 1 | 41 (23.2) | 23 (28.0) |
| 2 | 3 (1.7) | 2 (2.5) |
| Diagnostic method of CRC | ||
| Colonoscopic biopsy | 72 (87.8) | |
| Surgery | 10 (12.2) | |
| Diagnostic method of peritoneal | ||
| carcinomatosis | ||
| Cytology in ascites | 9 (11.0) | |
| CT image | 73 (89.0) | |
| Metastasis | ||
| Peritoneum only | 17 (20.7) | |
| Liver | 26 (31.7) | |
| Lung | 1 (1.2) | |
| Bone | 1 (1.2) | |
| Distant lymph node | 37 (45.2) | |
| Cytology | ||
| Positive | 0 (0.0) | 16 (21.1) |
| Negative | 168 (100.0) | 60 (78.9) |
Table 2.
Comparison of Diagnostic Parameters
| aCEA (ng/mL) | sCEA (ng/mL) | |
|---|---|---|
| Cut-off value | 3.885 | 8.635 |
| Sensitivity (%) | 96.3 | 84.2 |
| Specificity (%) PPV (%) | 100 100 | 91.3 81.0 |
| NPV (%) | 98.3 | 92.9 |
| AUC | 0.996 | 0.914 |
Table 3.
Factors Predicting the Occurrence of Peritoneal Carcinomatosis
OR, odds ratio; CI, confidence interval; aCEA, carcinoembryonic antigen in ascites; sCEA, carcinoembryonic antigen in serum; aCA19–9, carbohydrate antigen 19–9 in ascites; sCA19–9, carbohydrate antigen 19–9 in serum; SAAG, serum-ascites albumin gradient; protein A/S, ascites/serum concentration ratio of protein; LDH A/S, ascites/serum concentration ratio of lactate dehydrogenase.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download