Abstract
Detection of significant alloimmune response, which affects graft function and survival by effective immune monitoring, is critical for treatment decision making. However, there is no consensus regarding immune monitoring (IM) for kidney transplantation (flow KT) in Korea. The IM protocol may be affected by the level of immunological risk, the methods of desensitization and the availabilities of resources such as laboratory support and cost of tests. Questionnaire surveys designed to identify the current practices regarding immune monitoring of KT among transplant clinicians and clinical pathologists in Korea and eventually provide a basis for the establishment of harmonized immune monitoring guidelines in KT were administered as part of a Korean Society for Transplantation Sponsored Research Project. The survey results revealed significant variations in IM protocols and interpretation of tests affecting treatment decisions between institutes. Moreover, the results revealed a need to expand the histocompatibility tests into high resolution HLA typing in multiple loci and non-HLA antibody tests that facilitate the epitope analysis and eventually virtual crossmatching. The results of the questionnaire survey from clinical pathologists are addressing the urgent need for the standardization of interpretation and harmonization of results reporting in single antigen bead based HLA antibody identification. Finally, communication between clinicians and clinical pathologists to meet the clinical expectations regarding various immune monitoring tests is needed.
Go to : 
REFERENCES
1). Hart A., Smith JM., Skeans MA., Gustafson SK., Stewart DE., Cherikh WS, et al. OPTN/SRTR 2015 Annual Data Report: kidney. Am J Transplant. 2017. 17(Suppl 1):21–116.

2). Bartel G., Wahrmann M., Regele H., Kikic Z., Fischer G., Druml W, et al. Peritransplant immunoadsorption for positive crossmatch deceased donor kidney transplantation. Am J Transplant. 2010. 10:2033–42.

3). Morath C., Beimler J., Opelz G., Scherer S., Schmidt J., Macher-Goeppinger S, et al. Living donor kidney transplantation in crossmatch-positive patients enabled by peritransplant immunoadsorption and anti-CD20 therapy. Transpl Int. 2012. 25:506–17.

4). Morath C., Beimler J., Opelz G., Ovens J., Scherer S., Schmidt J, et al. An integrative approach for the transplantation of high-risk sensitized patients. Transplantation. 2010. 90:645–53.

5). Pei R., Lee JH., Shih NJ., Chen M., Terasaki PI. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation. 2003. 75:43–9.

6). Taylor CJ., Kosmoliaptsis V., Summers DM., Bradley JA. Back to the future: application of contemporary technology to long-standing questions about the clinical relevance of human leukocyte antigen-specific alloantibodies in renal transplantation. Hum Immunol. 2009. 70:563–8.

7). Cai J., Terasaki PI. Post-transplantation antibody monitoring and HLA antibody epitope identification. Curr Opin Immunol. 2008. 20:602–6.

8). Kosmoliaptsis V., Bradley JA., Sharples LD., Chaudhry A., Key T., Goodman RS, et al. Predicting the immunogenicity of human leukocyte antigen class I alloantigens using structural epitope analysis determined by HLAMatchmaker. Transplantation. 2008. 85:1817–25.

9). Duquesnoy RJ. HLAMatchmaker: a molecularly based algorithm for histocompatibility determination. I. Description of the algorithm. Hum Immunol. 2002. 63:339–52.

10). Dunn TB., Noreen H., Gillingham K., Maurer D., Ozturk OG., Pruett TL, et al. Revisiting traditional risk factors for rejection and graft loss after kidney transplantation. Am J Transplant. 2011. 11:2132–43.

11). Billen EV., Christiaans MH., Doxiadis II., Voorter CE., van den Berg-Loonen EM. HLA-DP antibodies before and after renal transplantation. Tissue Antigens. 2010. 75:278–85.

12). Dragun D., Muller DN., Brasen JH., Fritsche L., Nieminen-Kelha M., Dechend R, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005. 352:558–69.

13). Reinsmoen NL., Lai CH., Heidecke H., Haas M., Cao K., Ong G, et al. Anti-angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation. 2010. 90:1473–7.

14). Reinsmoen NL., Lai CH., Mirocha J., Cao K., Ong G., Naim M, et al. Increased negative impact of donor HLA-specific together with non-HLA-specific antibodies on graft outcome. Transplantation. 2014. 97:595–601.

15). Ming Y., Hu J., Luo Q., Ding X., Luo W., Zhuang Q, et al. Acute antibody-mediated rejection in presence of MICA-DSA and successful renal re-transplant with negative-MICA virtual crossmatch. PLoS One. 2015. 10:e0127861.

16). O Broin P., Hayde N., Bao Y., Ye B., Calder RB., de Boccardo G, et al. A pathogenesis-based transcript signature in donor-specific antibody-positive kidney transplant patients with normal biopsies. Genom Data. 2014. 2:357–60.
17). Kurian SM., Velazquez E., Thompson R., Whisenant T., Rose S., Riley N, et al. Orthogonal comparison of molecular signatures of kidney transplants with subclinical and clinical acute rejection: equivalent performance is agnostic to both technology and platform. Am J Transplant. 2017. 17:2103–16.

18). Choi JW., Kim YH., Oh JW. Comparative analyses of signature genes in acute rejection and operational tolerance. Immune Netw. 2017. 17:237–49.

19). Loupy A., Haas M., Solez K., Racusen L., Glotz D., Seron D, et al. The Banff 2015 Kidney Meeting Report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant. 2017. 17:28–41.

20). Haas M., Loupy A., Lefaucheur C., Roufosse C., Glotz D., Seron D, et al. The Banff 2017 Kidney Meeting report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018. 18:293–307.

21). Wiebe C., Gibson IW., Blydt-Hansen TD., Karpinski M., Ho J., Storsley LJ, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012. 12:1157–67.

22). Gloor JM., Cosio FG., Rea DJ., Wadei HM., Winters JL., Moore SB, et al. Histologic findings one year after positive crossmatch or ABO blood group incompatible living donor kidney transplantation. Am J Transplant. 2006. 6:1841–7.

23). Burns JM., Cornell LD., Perry DK., Pollinger HS., Gloor JM., Kremers WK, et al. Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. Am J Transplant. 2008. 8:2684–94.

24). Amico P., Honger G., Mayr M., Steiger J., Hopfer H., schaub S. Clinical relevance of pre-transplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation. 2009. 87:1681–8.

25). Hirai T., Kohei N., Omoto K., Ishida H., Tanabe K. Significance of low-level DSA detected by solid-phase assay in association with acute and chronic antibody-mediated rejection. Transpl Int. 2012. 25:925–34.

26). Tian J., Li D., Alberghini TV., Rewinski M., Guo N., Bow LM. Pre-transplant low level HLA antibody shows a composite poor outcome in long-term outcome of renal transplant recipients. Ren Fail. 2015. 37:198–202.

27). Tait BD., Susal C., Gebel HM., Nickerson PW., Zachary AA., Claas FH, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013. 95:19–47.

28). Korean Network for Organ Sharing (KONOS). 2017 Annual Data Report [Internet]. Seoul: KONOS;2017. Available from:. http://konos.go.kr.
29). Oh EJ., Park H., Park KU., Kang ES., Kim HS., Song EY. Interlaboratory Comparison of the results of lifecodes LSA class I and class II single antigen kits for human leukocyte antigen antibody detection. Ann Lab Med. 2015. 35:321–8.

30). Yu S., Kang ES., Park MH. A questionnaire survey of HLA crossmatch tests in Korea (2015). Lab Med Online. 2017. 7:147–56.

31). Dunn TB., Noreen H., Gillingham K., Maurer D., Ozturk OG., Pruett TL, et al. Revisiting traditional risk factors for rejection and graft loss after kidney transplantation. Am J Transplant. 2011. 11:2132–43.

32). Lee H., Min JW., Kim JI., Moon IS., Park KH., Yang CW, et al. Clinical significance of HLA-DQ antibodies in the development of chronic antibody-mediated rejection and allograft failure in kidney transplant recipients. Medicine (Baltimore). 2016. 95:e3094.

33). Bachelet T., Martinez C., Del Bello A., Couzi L., Kejji S., Guidicelli G, et al. Deleterious impact of donor-specific anti-HLA antibodies toward HLA-Cw and HLA-DP in kidney transplantation. Transplantation. 2016. 100:159–66.

34). Tagliamacco A., Cioni M., Comoli P., Ramondetta M., Brambilla C., Trivelli A, et al. DQ molecules are the principal stimulators of de novo donor-specific antibodies in nonsensitized pediatric recipients receiving a first kidney transplant. Transpl Int. 2014. 27:667–73.
35). Edathodu J., Varghese B., Alrajhi AA., Shoukri M., Nazmi A., Elgamal H, et al. Diagnostic potential of interferon-gamma release assay to detect latent tuberculosis infection in kidney transplant recipients. Transpl Infect Dis. 2017. 19:e12675.

36). Banas B., Steubl D., Renders L., Chittka D., Banas MC., Wekerle T, et al. Clinical validation of a novel enzyme-linked immunosorbent spot assay-based in vitro diagnostic assay to monitor cytomegalovirus-specific cell-mediated immunity in kidney transplant recipients: a multicenter, longitudinal, prospective, observational study. Transpl Int. 2018. 31:436–50.
37). Wang Z., Liu X., Lu P., Han Z., Tao J., Wang J, et al. Performance of the ImmuKnow assay in differentiating infection and acute rejection after kidney transplantation: a meta-analysis. Transplant Proc. 2014. 46:3343–51.

38). Potena L., Gaudenzi A., Chiereghin A., Borgese L., Brighenti A., Piccirilli G, et al. Quantiferon monitor assay identifies over-immunosuppressed patients with adverse outcomes after heart transplantation: towards the definition of a phenotype of immune frailty. J Heart Lung Transplant. 2018. 37(4 Suppl):S19–20.

39). Haas M., Sis B., Racusen LC., Solez K., Glotz D., Colvin RB, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014. 14:272–83.

40). Marsh SG., Albert ED., Bodmer WF., Bontrop RE., Dupont B., Erlich HA, et al. An update to HLA nomenclature, 2010. Bone Marrow Transplant. 2010. 45:846–8.

41). Amico P., Hirt-Minkowski P., Honger G., Gurke L., Mihatsch MJ., Steiger J, et al. Risk stratification by the virtual crossmatch: a prospective study in 233 renal transplantations. Transpl Int. 2011. 24:560–9.

42). Kosmoliaptsis V., Bradley JA., Peacock S., Chaudhry AN., Taylor CJ. Detection of immunoglobulin G human leukocyte antigen-specific alloantibodies in renal transplant patients using single-antigen-beads is compromised by the presence of immunoglobulin M human leukocyte antigen-specific alloantibodies. Transplantation. 2009. 87:813–20.

43). Schnaidt M., Weinstock C., Jurisic M., Schmid-Horch B., Ender A., Wernet D. HLA antibody specification using single-antigen beads: a technical solution for the prozone effect. Transplantation. 2011. 92:510–5.
44). Weinstock C., Schnaidt M. The complement-mediated prozone effect in the Luminex single-antigen bead assay and its impact on HLA antibody determination in patient sera. Int J Immunogenet. 2013. 40:171–7.

Go to : 
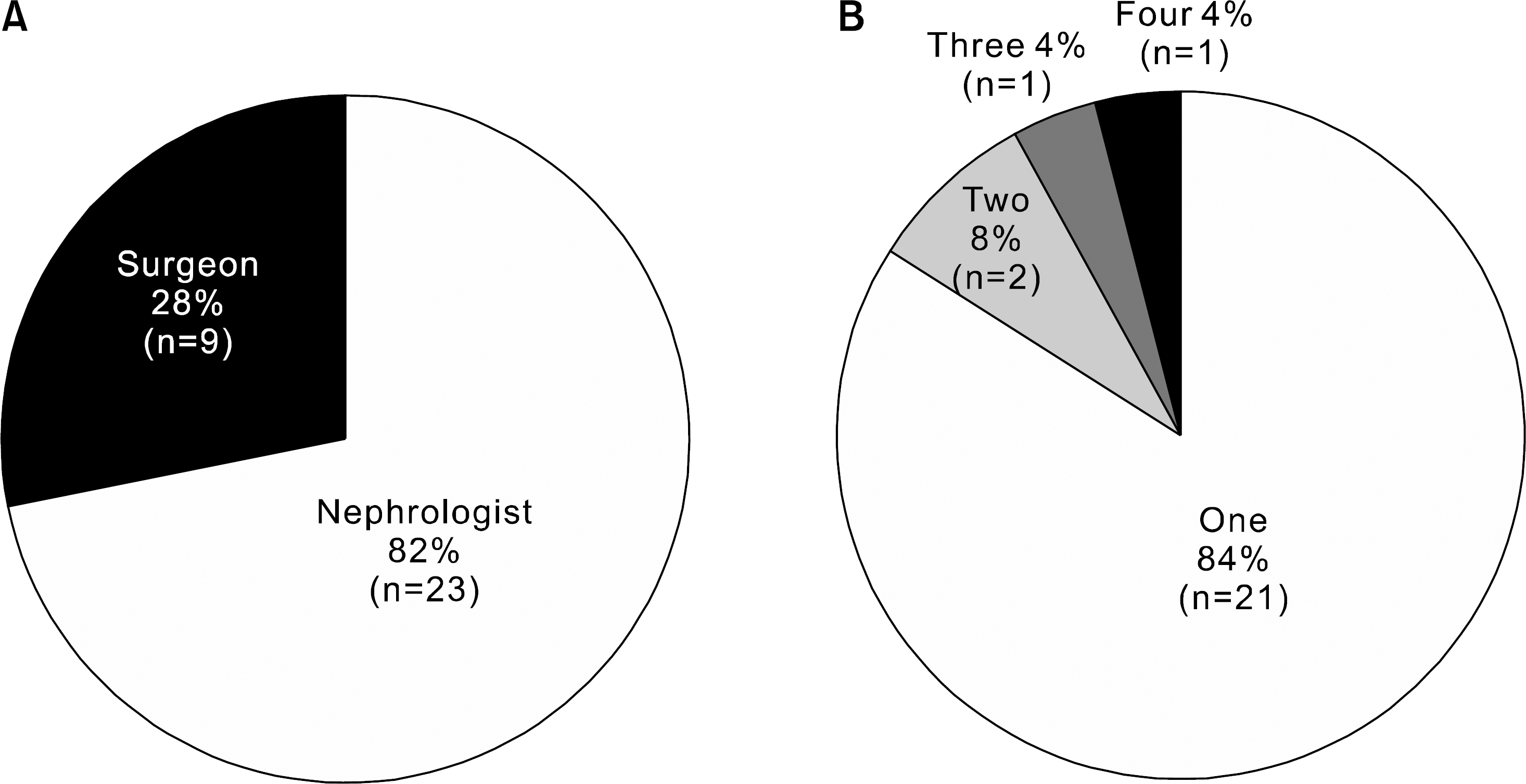 | Fig. 1.Proportions of transplant clinicians responded in questionnaire surveys according to (A) specialties and (B) number of clinicians per single institute. |
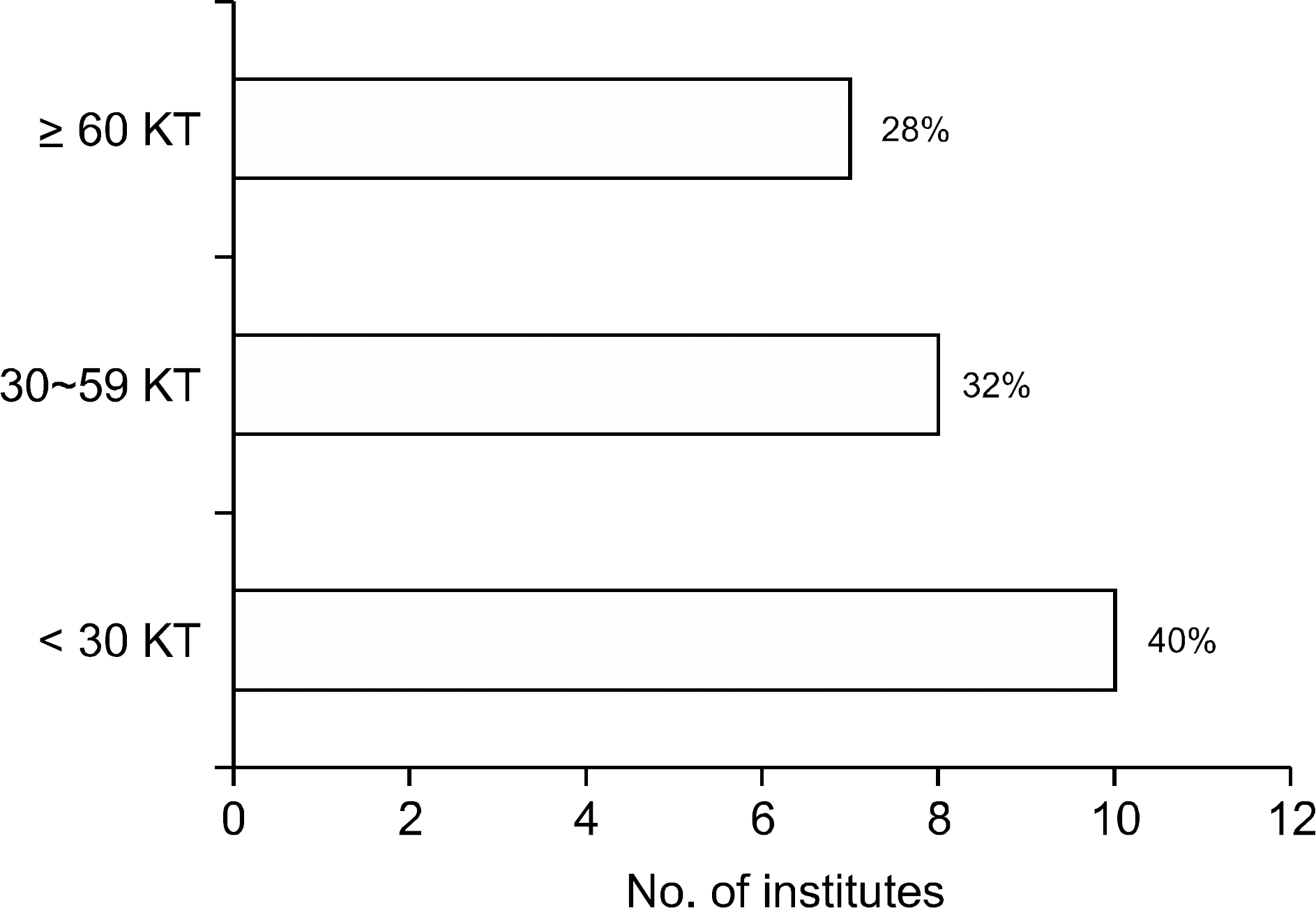 | Fig. 2.The number of kidney transplantation performed per year in institutes where the transplant clinicians responded to questionnaire survey. Abbreviation: KT, kidney transplantation. |
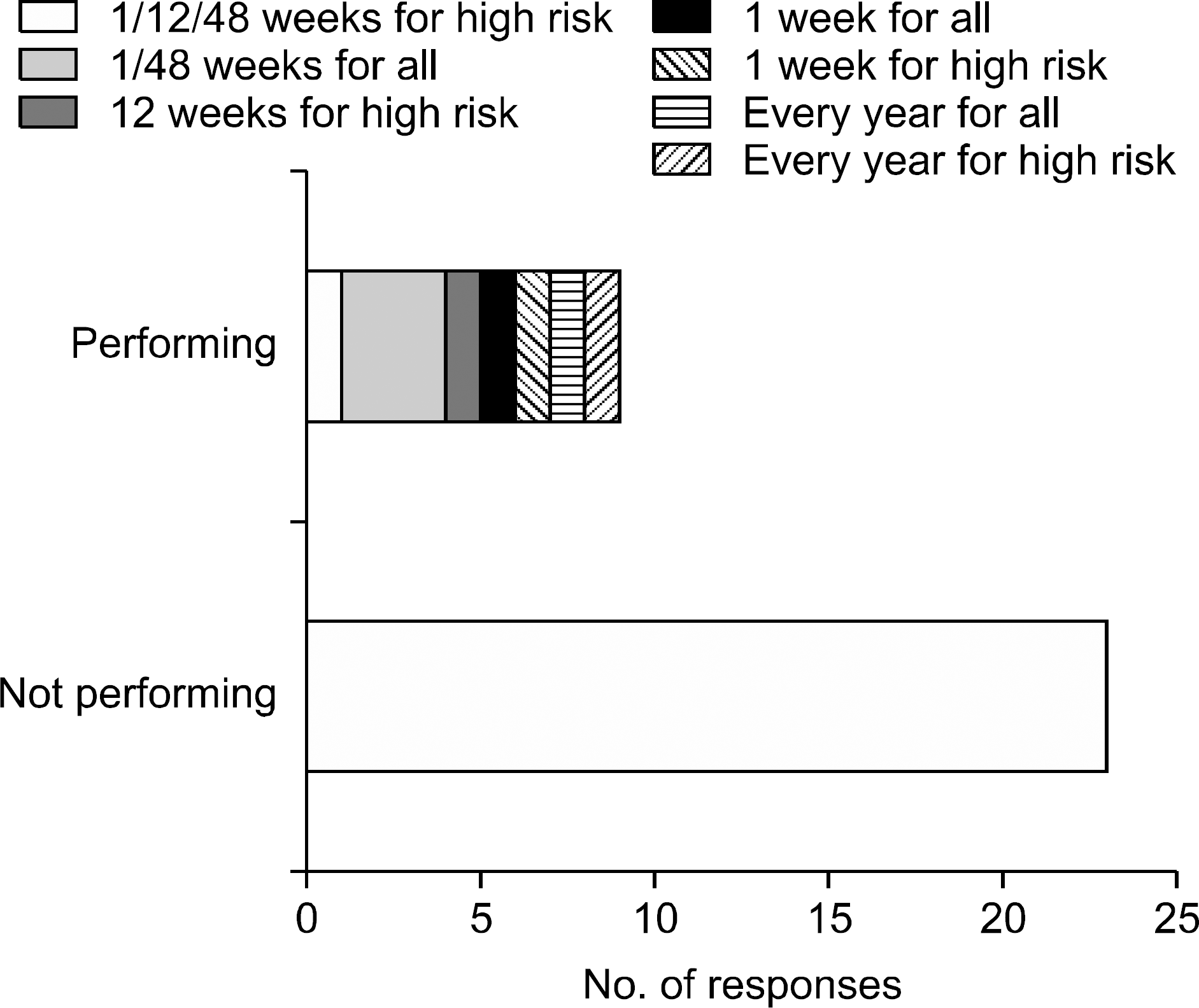 | Fig. 3.Status of performing protocol biopsies after kidney transplantation. Responses of 32 clinicians from 25 institutes. |
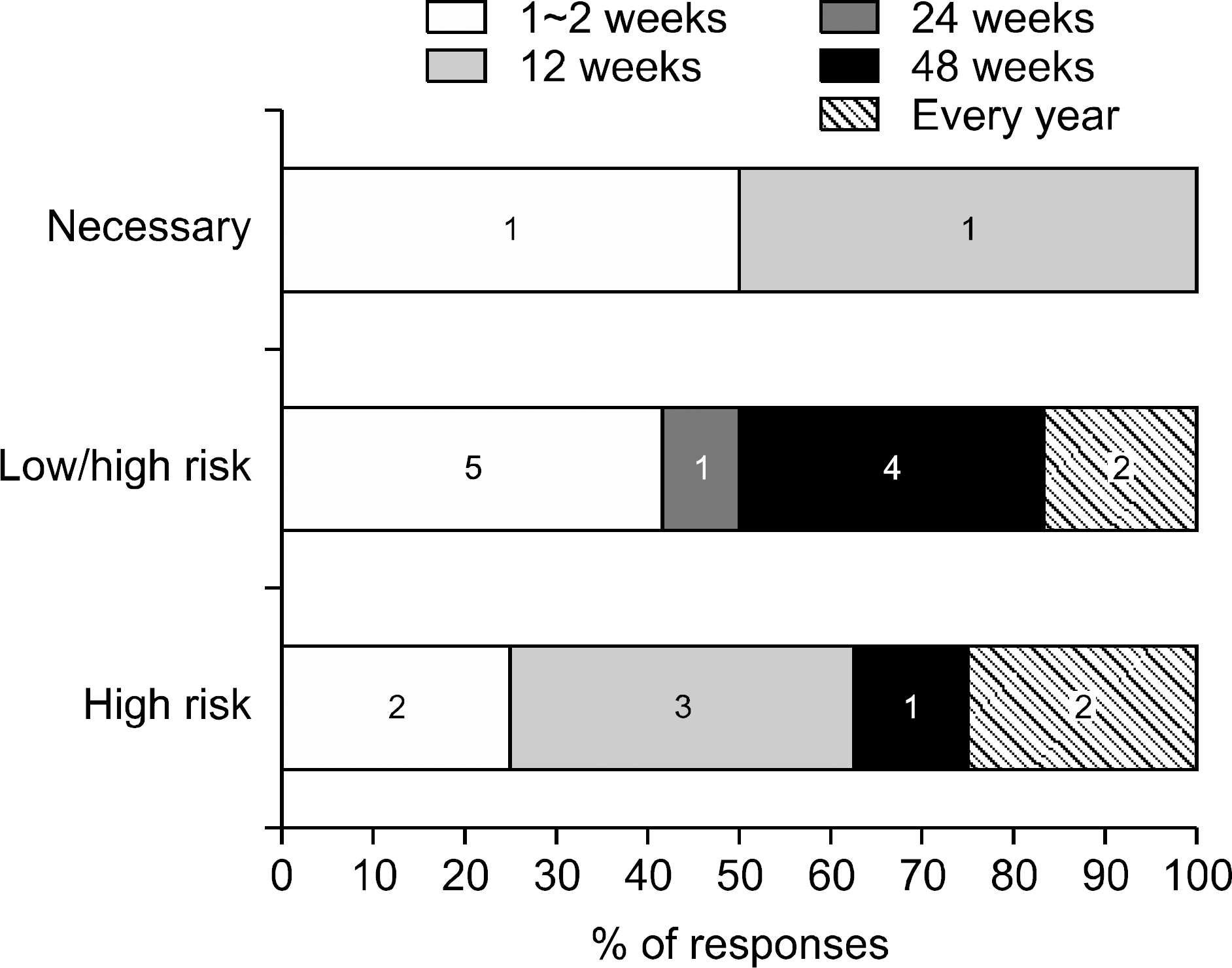 | Fig. 4.Status of performing protocol biopsies depending on the level of immunological risk after kidney transplantation. The protocol biopsies are being performed in recipients with high immunological risk or regardless of immunological risk in different time points. Two clinicians responded that protocol biopsies are necessary on 1∼2 week and 12 week posttransplant. Responses of 32 clinicians from 25 institutes. |
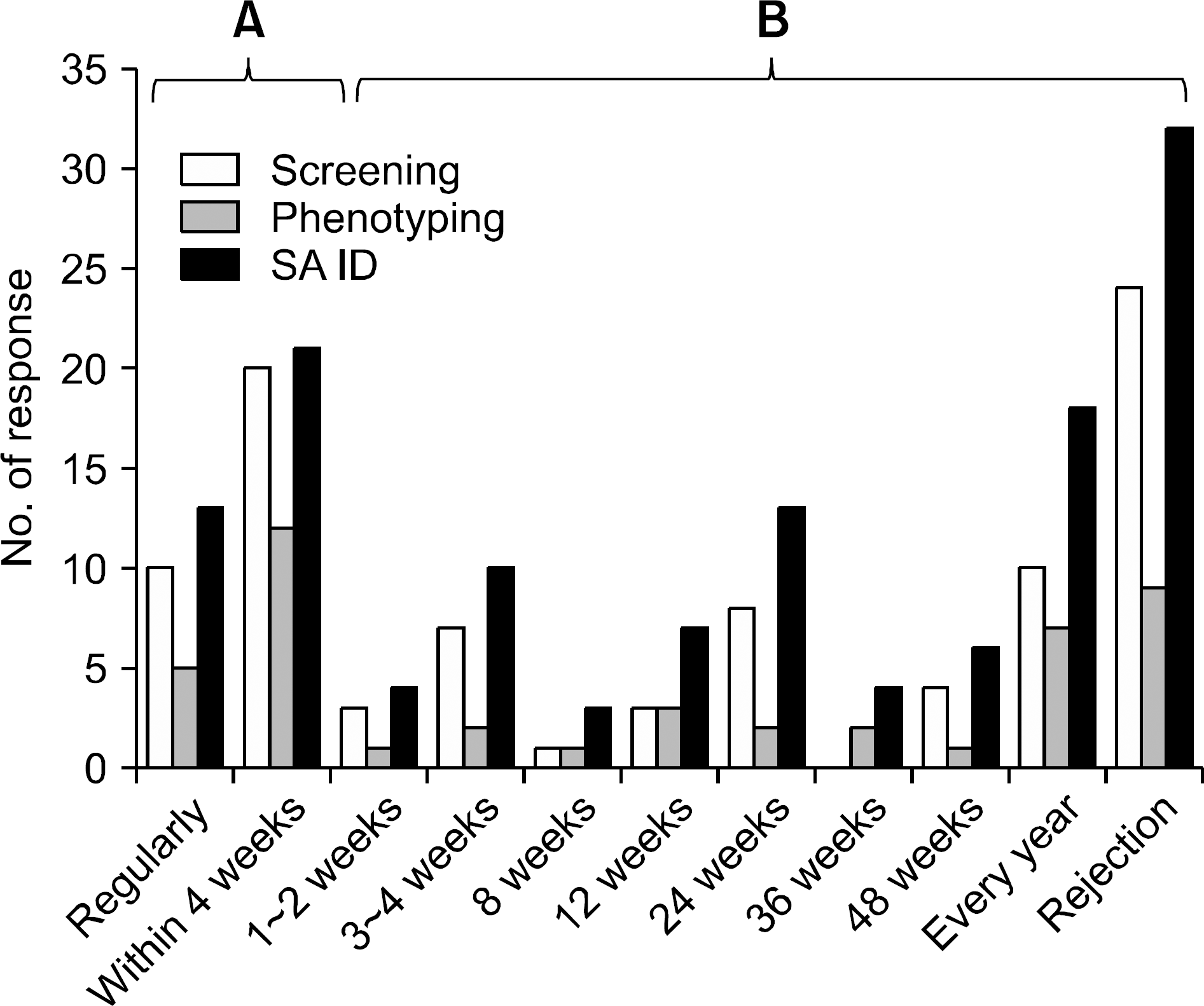 | Fig. 5.Status of performing HLA antibody tests in (A) pre- and (B) post-kidney transplantation. Responses of 32 clinicians from 25 institutes. Abbreviation: SA ID, single antigen identification. |
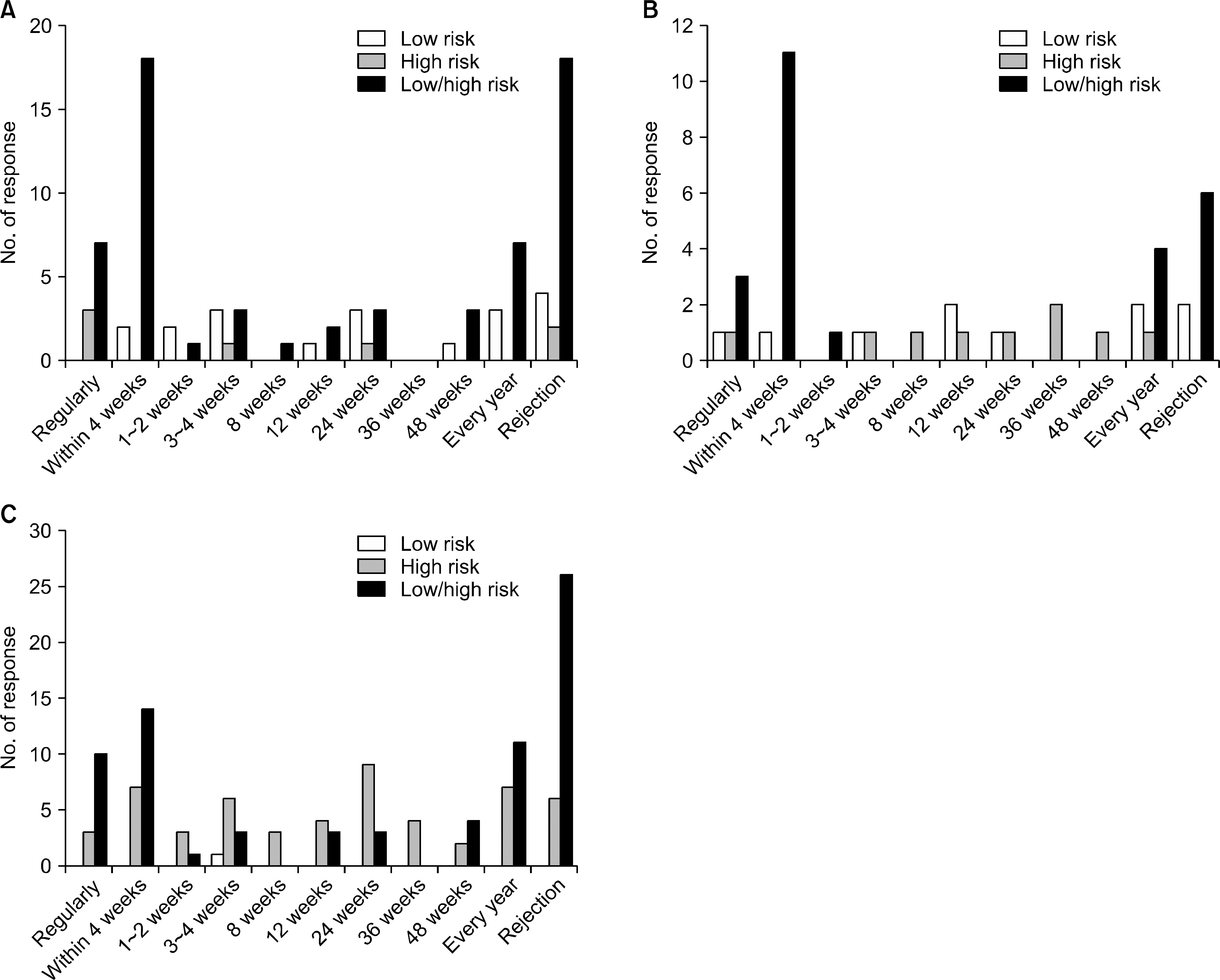 | Fig. 6.Status of performing HLA antibody tests depending on the level of immunological risk after kidney transplantation. (A) HLA antibody screening test, (B) HLA antibody phenotyping test, and (C) HLA antibody single antigen identification test. Abbreviation: HLA, human leukocyte antigen. |
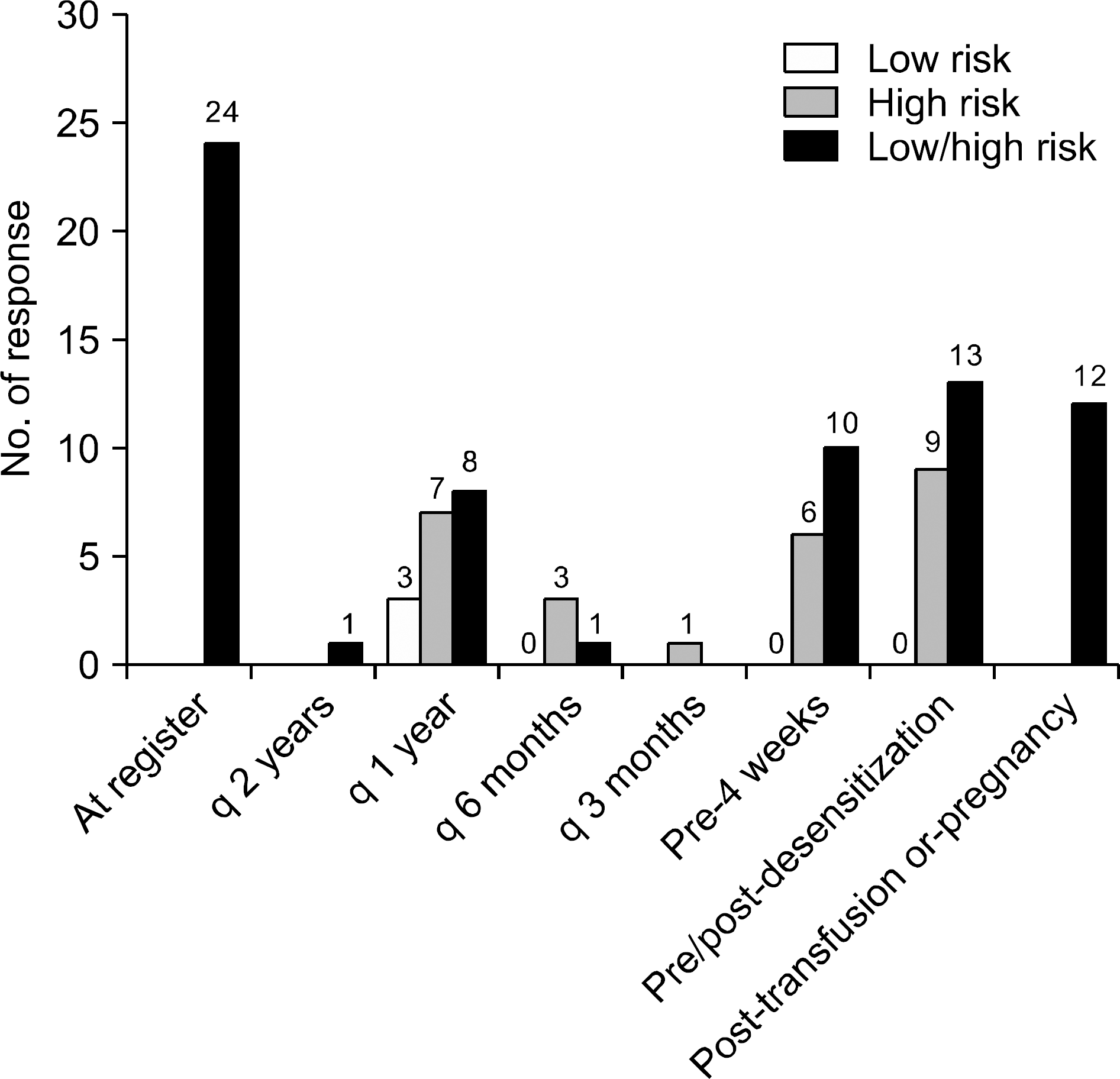 | Fig. 7.Suggested time points of HLA antibody monitoring test in patients waiting for kidney transplantation. Abbreviation: HLA, human leukocyte antigen. |
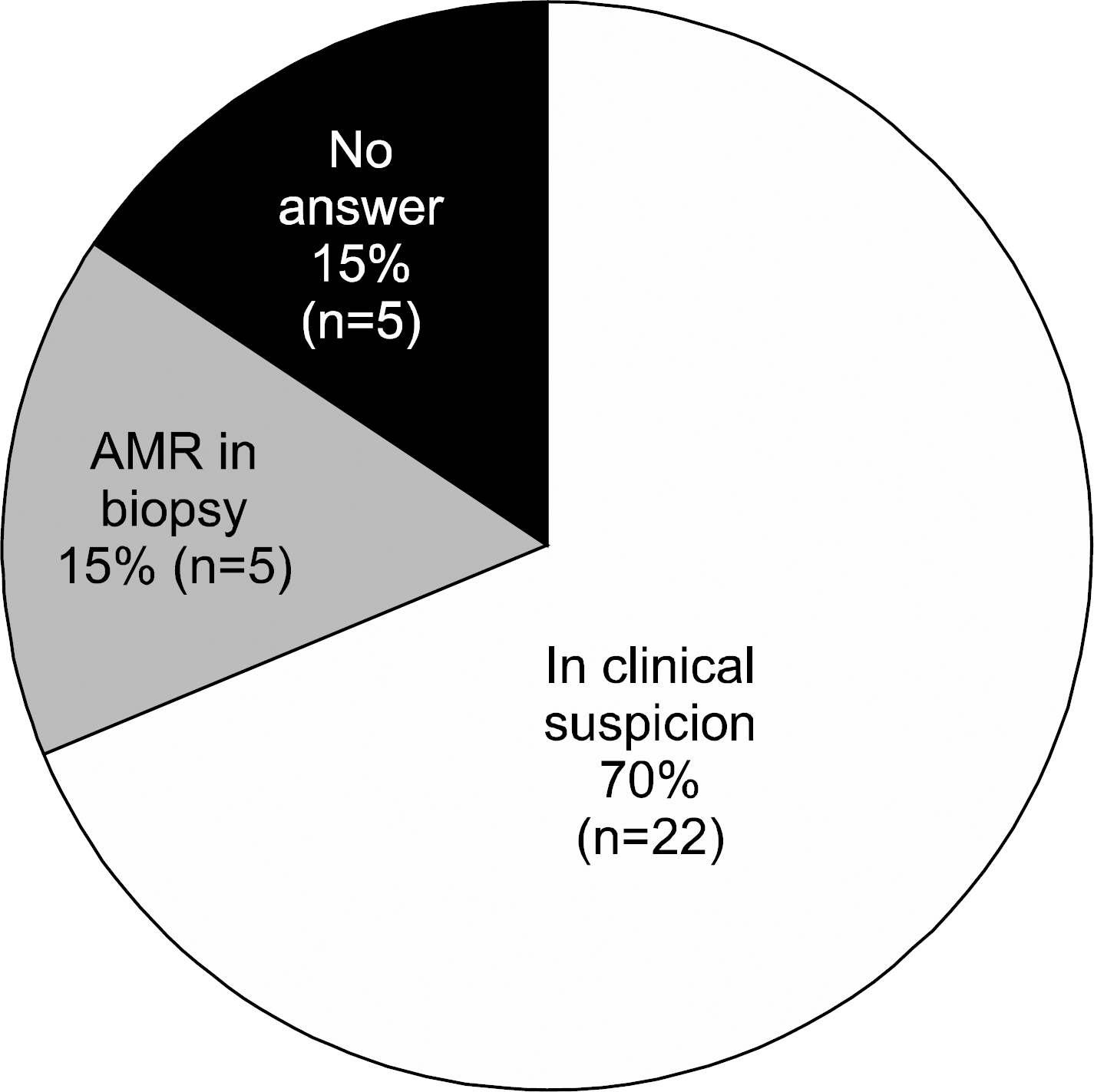 | Fig. 8.Indications of HLA antibody tests after kidney transplantation. Abbreviations: HLA, human leukocyte antigen; AMR, antibody mediated rejection. |
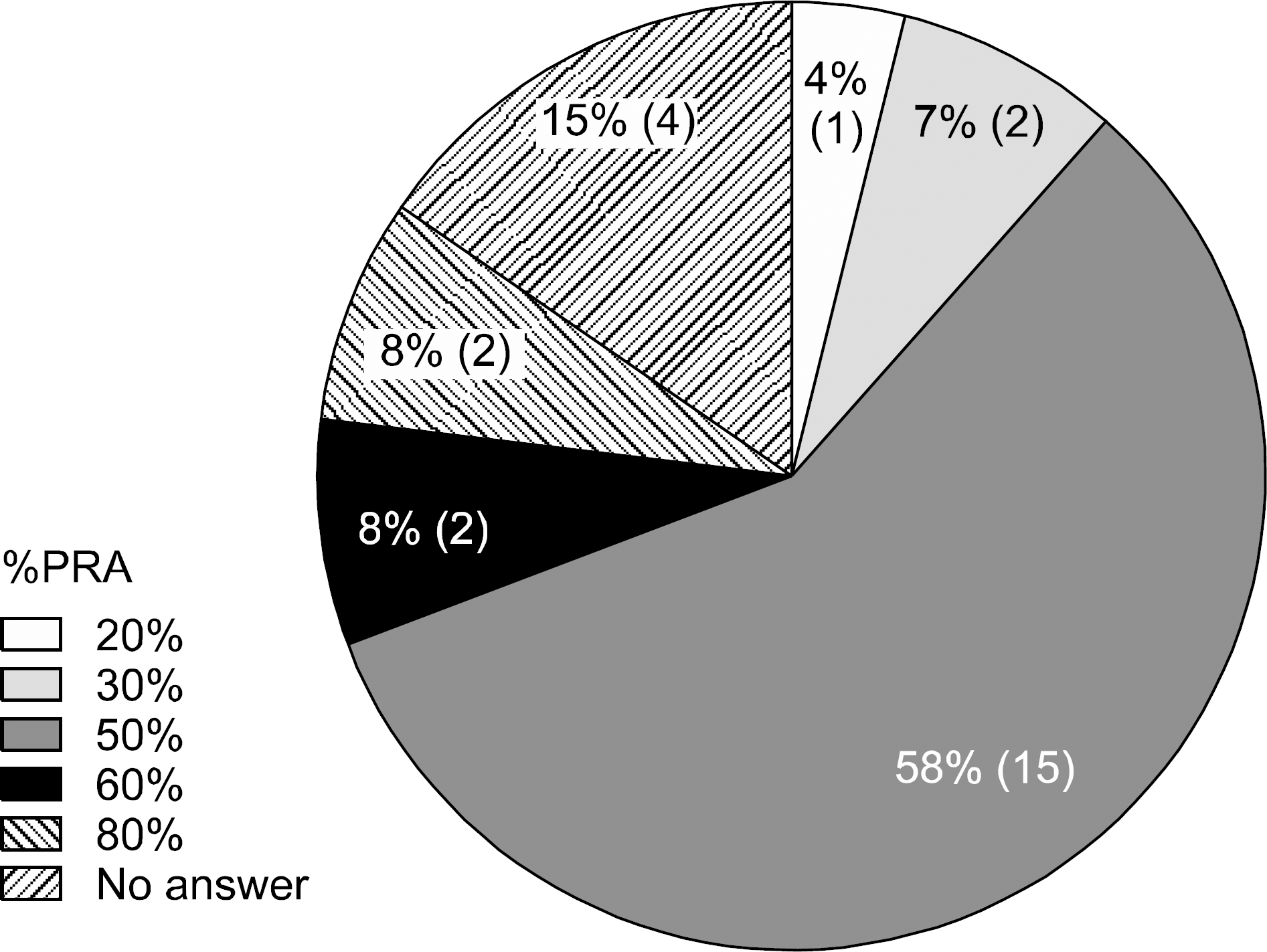 | Fig. 9.The threshold of %PRA to define high immunological risk in patients responded from 26 clinicians of 20 institutes. Abbreviation: PRA, percent reactive antibodies. |
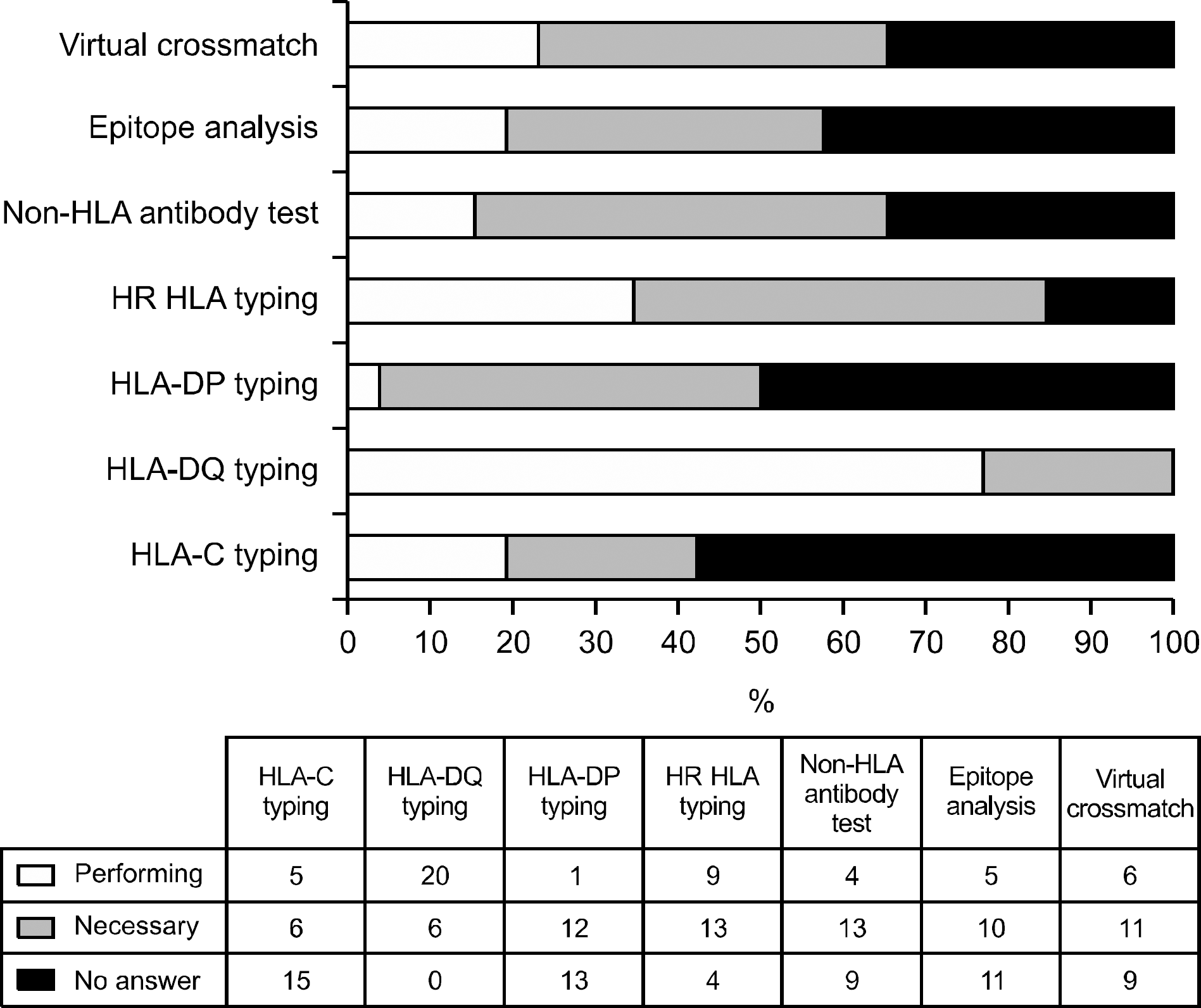 | Fig. 10.Clinicians opinions about the need of further tests on histocompatibility. Abbreviation: HLA, human leukocyte antigen. |
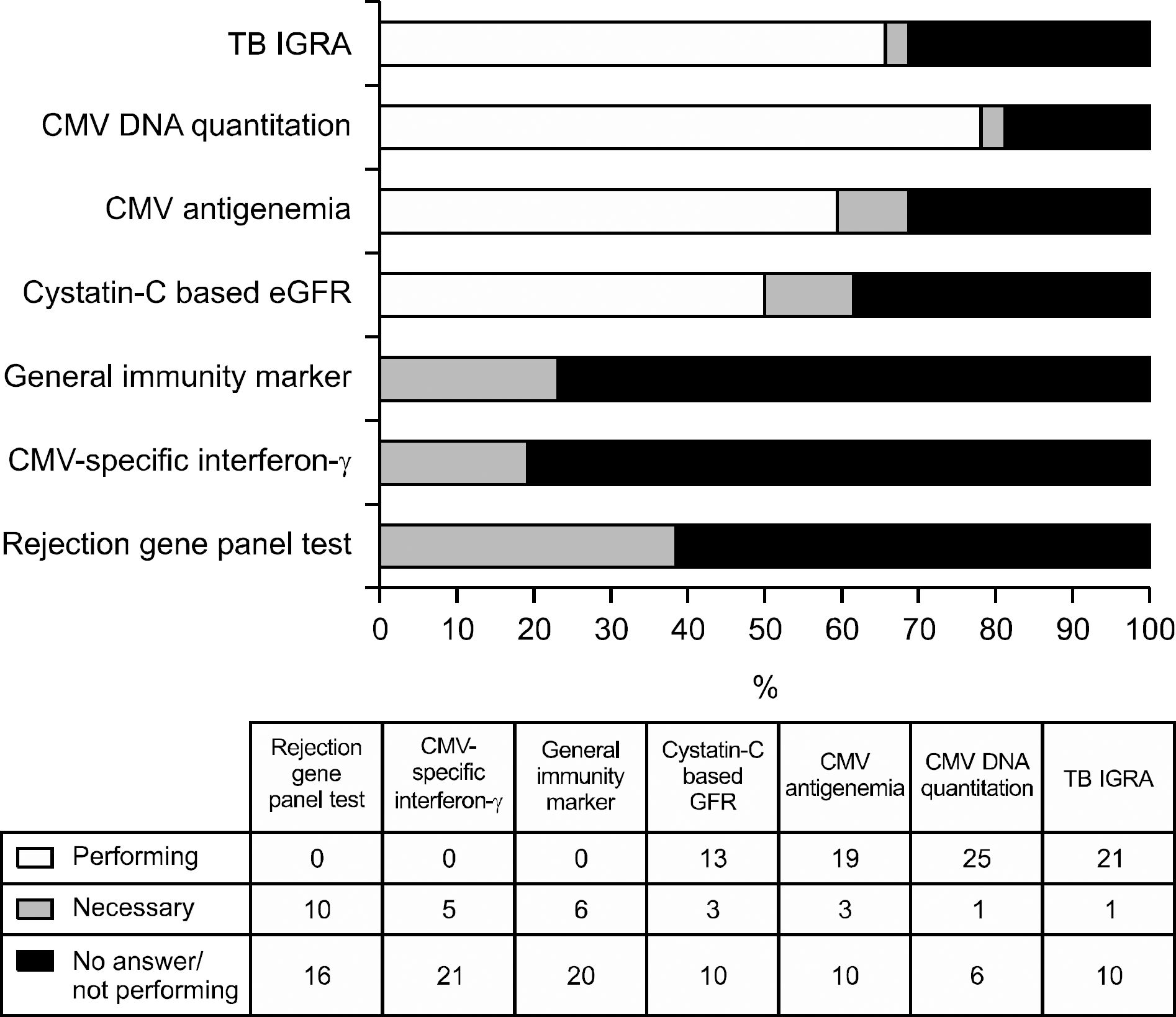 | Fig. 11.Clinicians opinions about the need of further immune monitoring tests. Abbreviations: IGRA, interferon gamma releasing assay; CMV, cytomegalovirus; eGFR, estimated glomerular filtration rate. |
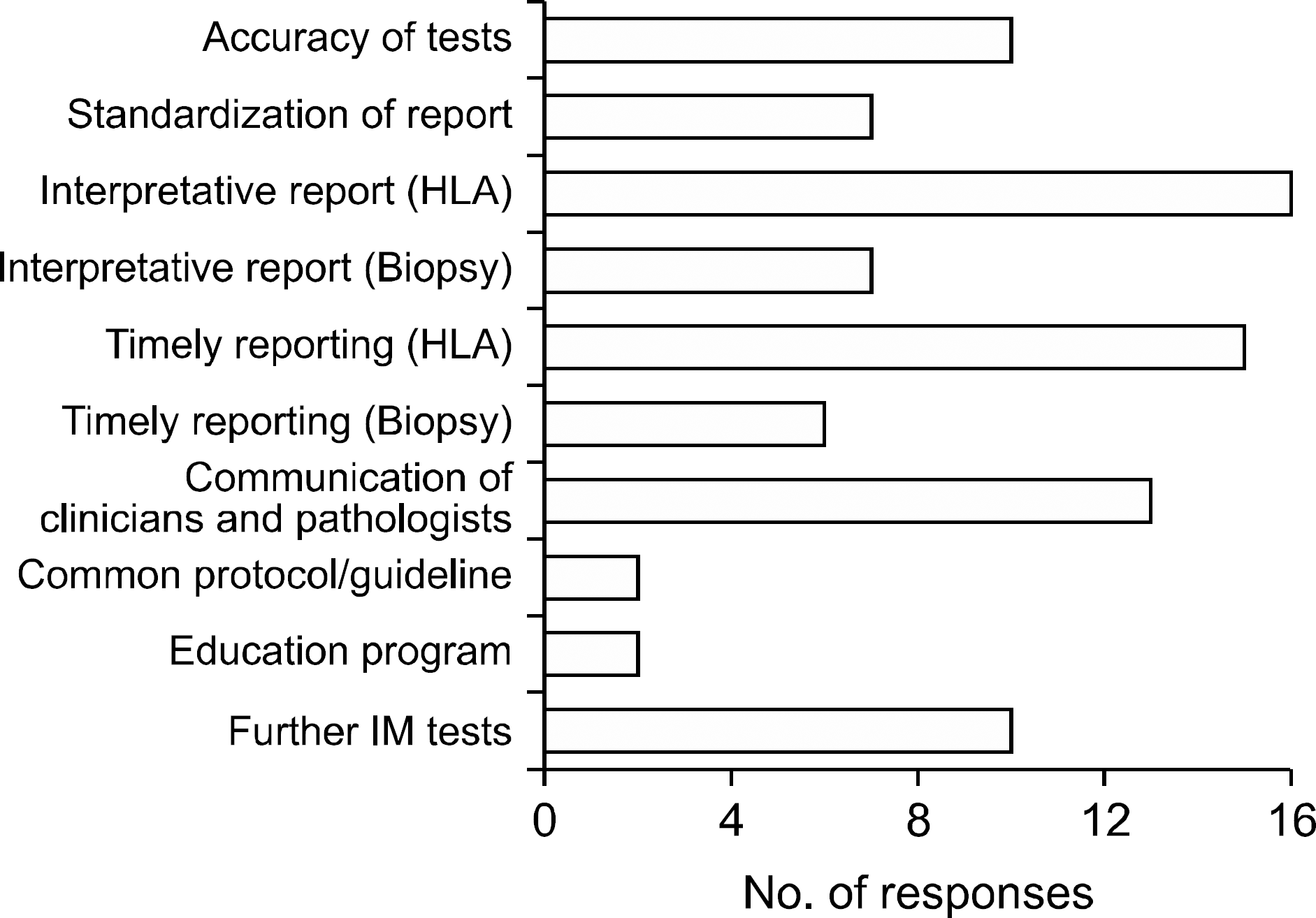 | Fig. 12.Clinicians opinions in areas which needs to be improved. Abbreviations: HLA, human leukocyte antigen; IM, immune monitoring. |
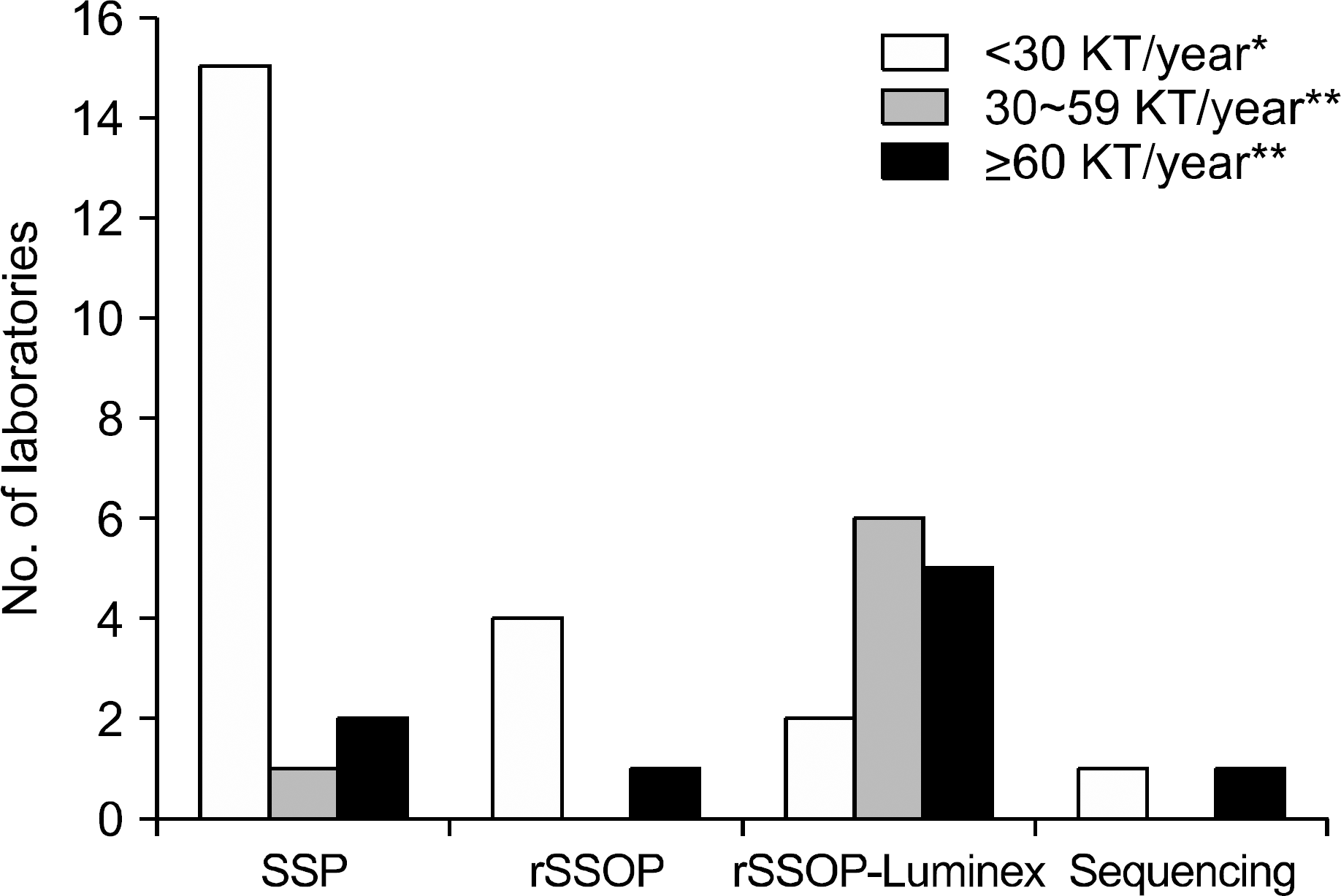 | Fig. 13.The principles of methods for HLA typing for kidney transplantation in responded laboratories according to the number of kidney transplantation performed per year in institutes. The data of three institutes referring HLA typing to outside labs are not included (∗) and the data of one institute of each performing HLA typing using two different methods are included (∗∗). Abbreviations: SSP, sequence-specific polymerase chain reaction; rSSOP, reverse sequence-specific oligonucleotide probe hybridization. |
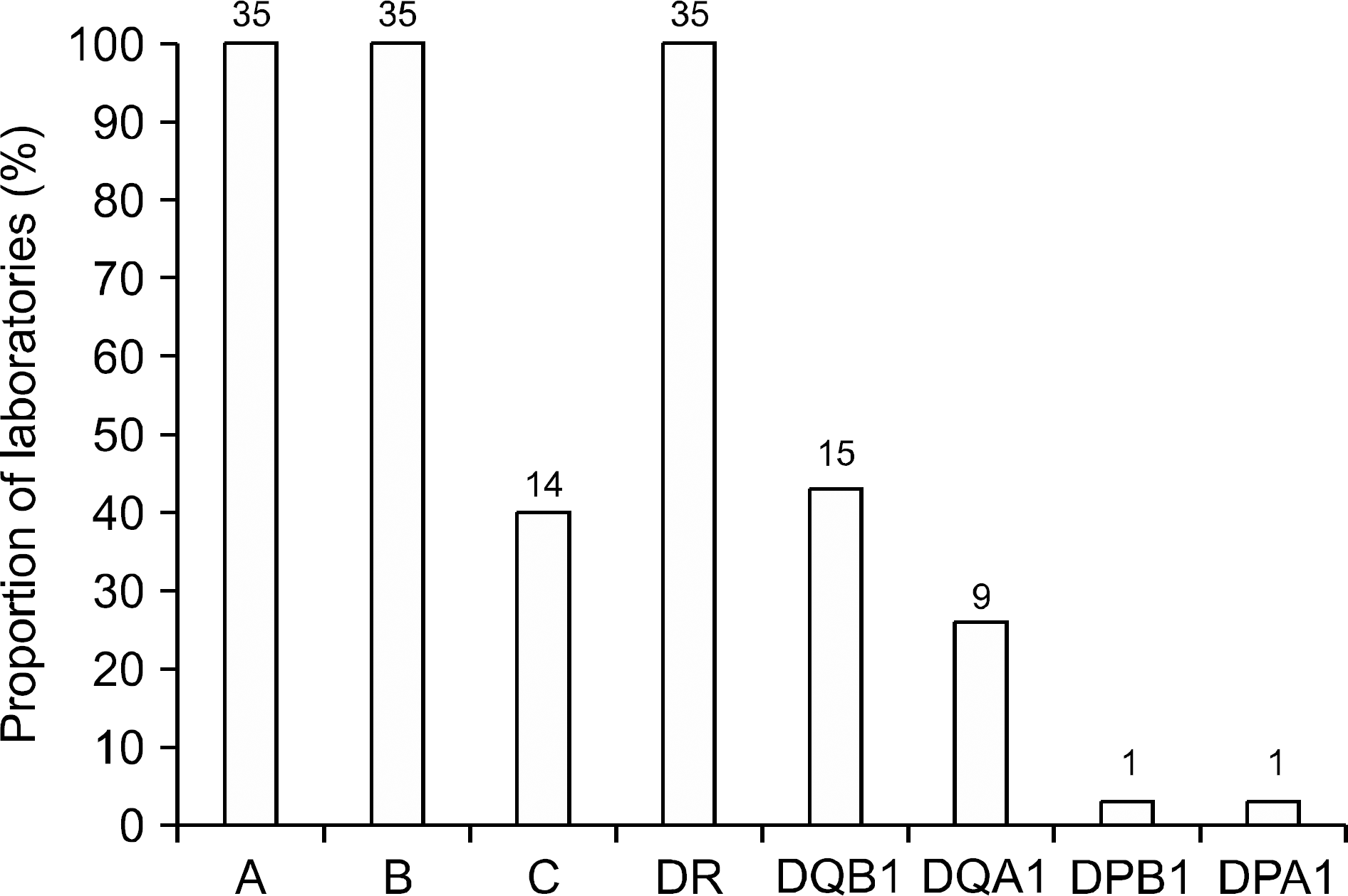 | Fig. 14.HLA loci tested for kidney transplantation in responding laboratories. Abbreviation: HLA, human leukocyte antigen. |
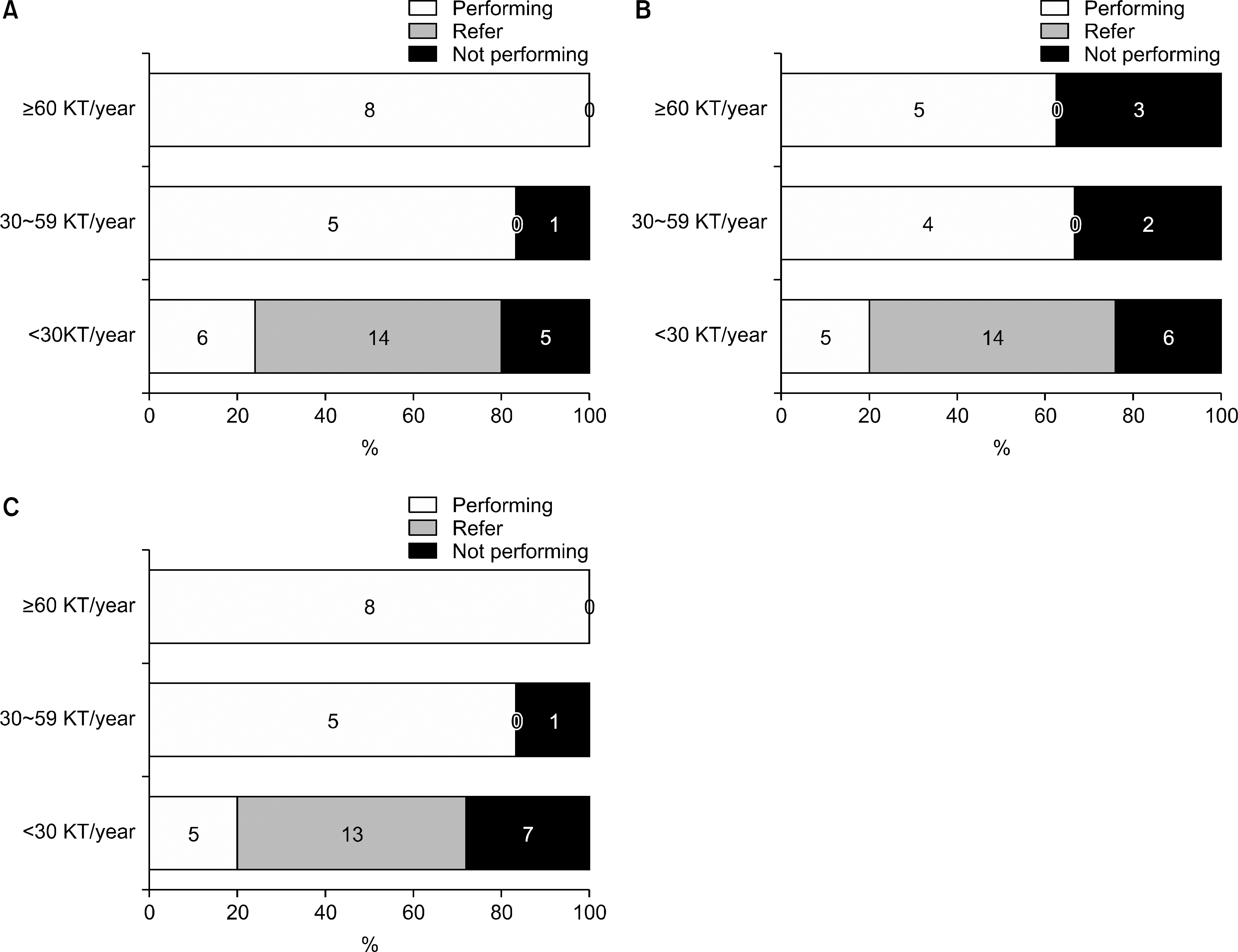 | Fig. 15.The status of performing HLA antibody tests in responded laboratories according to the number of kidney transplantation performed per year in institutes. (A) Screening test, (B) Phenotyping, (C) Single antigen bead identification. Abbreviation: HLA, human leukocyte antigen. |
Table 1.
Clinicians opinions about the impact of immune monitoring tests on deciding treatment strategies regardless of current practices
Table 2.
The threshold of DSA MFI in each locus to define high immunological risk in patients responded from 32 clinicians of 25 institutes
| Threshold of DSA MFI | AB (n=26) | DR (n=26) | DQ (n=22) |
|---|---|---|---|
| 1,000 | 6 | 7 | 6 |
| 2,000∼3,000 | 8 | 7 | 7 |
| 4,000 | 1 | 1 | 1 |
| 5,000 | 9 | 9 | 7 |
| 10,000 | 2 | 2 | 1 |
| c |
Table 3.
The format and content of single antigen bead based HLA antibody identification result reporting in different HLA laboratories (n=18)




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download