1. Lau C, Mar t, Bunnapradist S. Management of renal dysfunction in patients receiving a liver transplant. Clin Liver Dis. 2011; 15:807–820. PMID:
22032530.

2. Matuszkiewicz-Rowińska J, Wieliczko M, Małyszko J. Renal replacement therapy before, during, and after orthotopic liver transplantation. Ann Transplant. 2013; 18:248–255. PMID:
23792528.

3. Ikegami T, Shirabe K, Soejima Y, Taketomi A, Yoshizumi T, Uchiyama H, et al. The impact of renal replacement therapy before or after living donor liver transplantation. Clin Transplant. 2012; 26:143–148. PMID:
21447144.

4. Nadim MK, Annanthapanyasut W, Matsuoka L, Appachu K, Boyajian M, Ji L, et al. Intraoperative hemodialysis during liver transplantation: a decade of experience. Liver Transpl. 2014; 20:756–764. PMID:
24634344.

5. Parmar A, Bigam D, Meeberg G, Cave D, Townsend DR, Gibney RT, et al. An evaluation of intraoperative renal support during liver transplantation: a matched cohort study. Blood Purif. 2011; 32:238–248. PMID:
21829016.

6. Townsend DR, Bagshaw SM, Jacka MJ, Bigam D, Cave D, Gibney RT. Intraoperative renal support during liver transplantation. Liver Transpl. 2009; 15:73–78. PMID:
19109832.

7. Akhoundi A, Singh B, Vela M, Chaudhary S, Monaghan M, Wilson GA, et al. Incidence of adverse events during continuous renal replacement therapy. Blood Purif. 2015; 39:333–339. PMID:
26022612.

8. Dawwas MF, Lewsey JD, Watson CJ, Gimson AE. UK, Ireland Liver Transplant Audit. The impact of serum potassium concentration on mortality after liver transplantation: a cohort multicenter study. Transplantation. 2009; 88:402–410. PMID:
19667945.

9. Shangraw RE. Metabolic issues in liver transplantation. Int Anesthesiol Clin. 2006; 44:1–20.

10. Kamath PS, Kim WR. Advanced Liver Disease Study Group. The model for endstage liver disease (MELD). Hepatology. 2007; 45:797–805. PMID:
17326206.

11. Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964; 1:1–85. PMID:
4950264.
12. Bang SR, Ahn HJ, Kim GS, Yang M, Gwak MS, Ko JS, et al. Predictors of high intraoperative blood loss derived by simple and objective method in adult living donor liver transplantation. Transplant Proc. 2010; 42:4148–4150. PMID:
21168648.

13. De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010; 362:779–789. PMID:
20200382.

14. De Backer D, Creteur J, Silva E, Vincent JL. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is best? Crit Care Med. 2003; 31:1659–1667. PMID:
12794401.

15. Russell JA, Walley KR, Singer J, Gordon AC, Hébert PC, Cooper DJ, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008; 358:877–887. PMID:
18305265.

16. Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006; 6:2651–2659. PMID:
16939515.

17. Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, et al. Renal replacement therapy and orthotopic l iver transplant ation: the role of continuous veno-venous hemodialysis. Transplantation. 2001; 71:1424–1428. PMID:
11391230.
18. Salord F, Bailly MP, Gaussorgues P, Workineh S, Pouyet M, Robert D. Continuous arteriovenous haemodialysis during emergency hepatic retransplantation: two case reports. Intensive Care Med. 1990; 16:330–331. PMID:
2212260.

19. Douthitt L, Bezinover D, Uemura T, Kadry Z, Shah RA, Ghahramani N, et al. Perioperative use of continuous renal replacement therapy for orthotopic liver transplantation. Transplant Proc. 2012; 44:1314–1317. PMID:
22664007.

20. Vitin A, Muczynski K, Bakthavatsalam R, Martay K, Dembo G, Metzner J. Treatment of severe lactic acidosis during the preanhepatic stage of liver transplant surgery with intraoperative hemodialysis. J Clin Anesth. 2010; 22:466–472. PMID:
20868970.

21. Sedra AH, Strum E. The role of intraoperative hemodialysis in liver transplant patients. Curr Opin Organ Transplant. 2011; 16:323–325. PMID:
21543980.

22. Bellomo R, Harris C, Kang Y, Daniel E, Fung JJ, Bronsther O. Combined veno-venous bypass and high volume hemofiltration during orthotopic liver transplantation. ASAIO J. 1993; 39:954–956. PMID:
8123934.

23. Agopian VG, Dhillon A, Baber J, Kaldas FM, Zarrinpar A, Farmer DG, et al. Liver transplantation in recipients receiving renal replacement therapy: outcomes analysis and the role of intraoperative hemodialysis. Am J Transplant. 2014; 14:1638–1647. PMID:
24854341.

24. Gruber M, Breu A, Frauendorf M, Seyfried T, Hansen E. Washing of banked blood by three different blood salvage devices. Transfusion. 2013; 53:1001–1009. PMID:
22897672.

25. Vraets A, Lin Y, Callum JL. Transfusion-associated hyperkalemia. Transfus Med Rev. 2011; 25:184–196. PMID:
21498041.

26. Glanemann M, Langrehr J, Kaisers U, Schenk R, Müller A, Stange B, et al. Postoperative tracheal extubation after orthotopic liver transplantation. Acta Anaesthesiol Scand. 2001; 45:333–339. PMID:
11207470.

27. Snowden CP, Hughes T, Rose J, Roberts DR. Pulmonary edema in patients after liver transplantation. Liver Transpl. 2000; 6:466–470. PMID:
10915170.

28. Lin YH, Cai ZS, Jiang Y, Lü LZ, Zhang XJ, Cai QC. Perioperative risk factors for pulmonary complications after liver transplantation. J Int Med Res. 2010; 38:1845–1855. PMID:
21309501.

29. Feltracco P, Carollo C, Barbieri S, Pettenuzzo T, Ori C. Early respiratory complications after liver transplantation. World J Gastroenterol. 2013; 19:9271–9281. PMID:
24409054.

30. Wi W, Hahm TS, Kim GS. A case series on simultaneous l iver and kidney transplantation: do we need intraoperative renal replacement therapy? Korean J Anesthesiol. 2017; 70:467–476. PMID:
28794844.
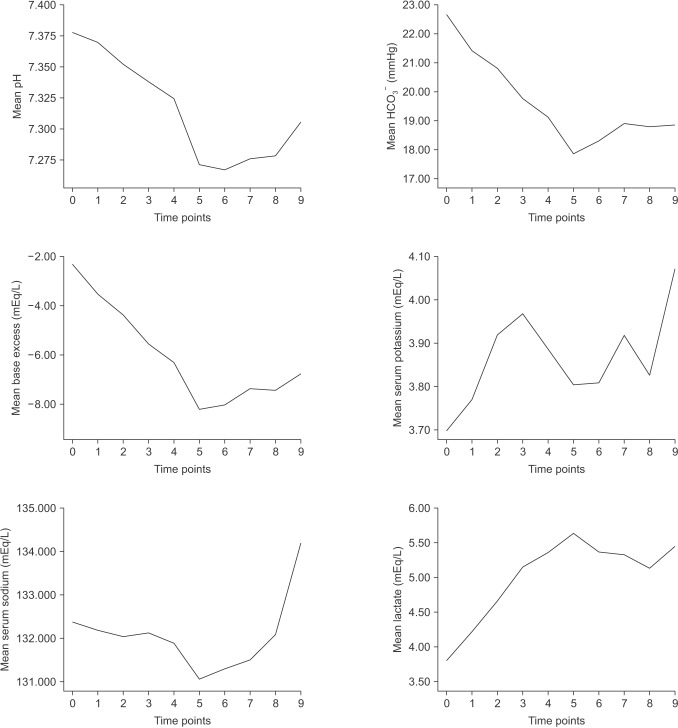
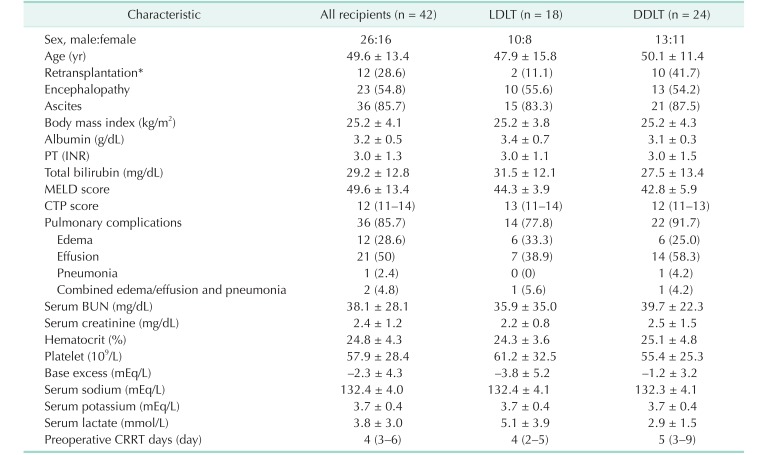
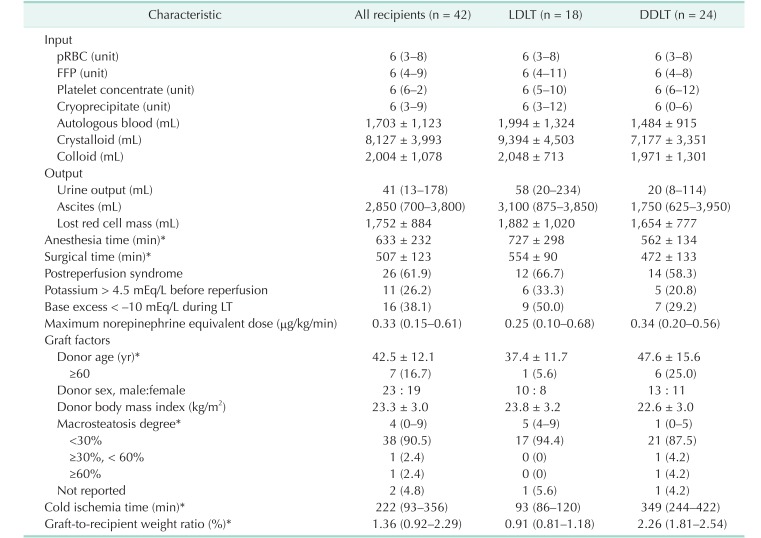
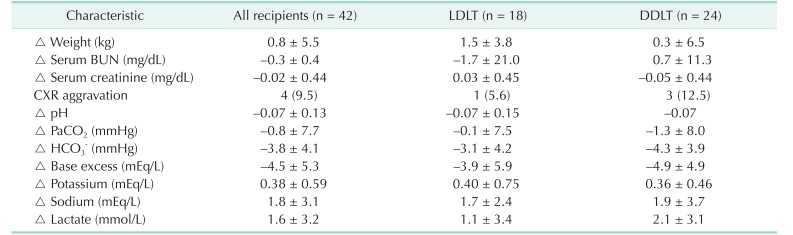




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download